Abstract
Background: The catalyst for this analysis was a statement by Dr. Magnus Fall at a meeting of invited experts in 2020 of IC/BPS (interstitial cystitis/bladder pain syndrome): “A paradigm shift in the understanding of IC/BPS is urgent.” This review analyses such a paradigm shift. Summary: The paradigm shift began with the serendipitous cure of Hunner’s lesion in a woman with a diagnosis of posterior fornix syndrome (PFS) and uterine prolapse. Retrospective analysis of surgical data from eight PFS studies reporting pain and urge cure found PFS was consistent with IC/BPS definitions. No Hunner’s lesions/ulcers were reported in the eight studies. Anatomical pathways for urge/frequency were consistent with Tanagho’s descriptions of normal micturition (albeit prematurely activated); pathways for abnormal emptying/retention were consistent with inability of pelvic muscles to open the posterior urethral wall prior to micturition; pathways for pelvic pain were consistent with de novo impulses from pelvic visceral plexuses caused by unsupported USLs (uterosacral ligaments). Key Messages: As PFS and IC/BPS have similar symptoms, USL repair of prolapse can potentially deliver improvement/cure for urge and pain symptoms, provided diagnostic PFS criteria (e.g., speculum test) for PFS are met. Two hypotheses explain inflammatory end-organ responses observed in Hunner’s IC/BPS and offer new research directions.
Plain Language Summary
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a bladder condition, which consists of pelvic pain and “urge to go,” with sometimes an ulcer in the bladder called Hunner’s ulcer. At present, interstitial cystitis is considered incurable with no known cause. This paper concerns a serendipitous discovery of cure of Hunner’s lesion by repairing the suspensory ligaments of the uterus (uterosacral) in a 73-year-old woman for a different condition, prolapse, urge, pain, and bladder emptying problems, known as the “posterior fornix syndrome” (PFS). High cure rates for these symptoms were confirmed in eight different studies comprising hundreds of women with PFS. The question this paper analyses is, “are PFS and IC/BPS one and the same condition in some women who have IC/BPS?” If so, then IC/BPS is potentially curable by repair of the uterosacral ligaments.
Introduction
The inspiration for this work was a statement by an eminent veteran expert in interstitial cystitis/bladder pain syndrome (IC/BPS) science, Dr Magnus Fall: “A paradigm shift in the understanding of IC/BPS is urgent” [1]. The context was a debate within ESSIC (the IC/BPS society) about whether Hunner’s lesion (HL) or ulcer (HU) were a separate disease entity from non-Hunner’s IC/BPS [1]. We interpreted his statement as a “cri de coeur” for a commonly acknowledged crisis summarized (frustatingly) by an erudite review on IC/BPS immunology as [2]: “Despite multiple clinical phenotypes and hypotheses for causation, and multiple failures of therapeutic trials, a validated aetiology for the disorder has yet to be identified.” The recent report of the ICI (International Consultation on Incontinence) confirms this dilemma [3]: “The aetiology of IC/BPS remains a wide-open field for investigation.” IC/BPS is defined as, “A 6-month duration of chronic pelvic pain (CPP), pressure, or discomfort perceived to be related to the urinary bladder accompanied by at least one other urinary symptom such as persistent urge to void or frequency. Confusable diseases as the cause of the symptoms must be excluded” [3].
The new direction for the causation of IC/BPS, which we present, is the posterior fornix syndrome (PFS)1, which has existed as a parallel universe since 1993 [4]. PFS was part of the 2nd iteration of the Integral Theory of Female Urinary Incontinence (IT) [5], emphasizing altered ligament collagen as a key causative factor in pelvic floor dysfunction. PFS is defined as “predictably co-occurring CPP, urge, frequency, nocturia emptying difficulties/urinary retention, caused by uterosacral ligament (USL) laxity, and cured by USL repair” [4, 5]. A strong connection between PFS and IC/BPS was only brought to light in 2021, by a serendipitous, histologically validated cure of HL in a 73-year-old German woman treated strictly according to the diagnostic and surgical protocols for PFS by Dr. Kay Scheffler, a double-trained gynaecologist/urologist [6].
Importance of the Scheffler Discovery
It overturned all previous concepts to do with IC/BPS as defined by the ICS (International Continence Society) and ESSIC (European Society for the Study of Interstitial Cystitis) [1‒3]: with IC/BPS having no known causation and cure. Quoting Karl Popper’s statement on hypotheses and theories: one validated case can destroy all previous concepts2.
In his 1962 book The Structure of Scientific Revolutions, American physicist and philosopher, Thomas Kuhn, characterized a paradigm shift as a revolution that challenges and ultimately takes the place of a prevailing scientific framework. Such challenges arise when the dominant paradigm, under which commonly accepted science operates, is found to be incompatible or insufficient for new data or findings, facilitating the adoption of a revised or completely new theory or paradigm [7].
The aim of this review was not to “challenge and ultimately take the place of a prevailing scientific framework.” Instead, it aims to summarize the multiple scientific contributions by many researchers to the anatomical aetiopathogeneses [5] behind the PFS symptoms (CPP, urge, frequency, nocturia, abnormal emptying) and the reported surgical and non-surgical improvement or cure. The aetiopathogenesis of these symptoms is key to our assertion that if women diagnosed as non-Hunner’s IC/BPS fit the diagnostic criteria of PFS, their condition can be considered similar to PFS. As such, it is potentially curable by USL repair.
We emphasize, and it is evident in the text of our paper, that we are not claiming that PFS is the cause of all non-H IC/BPS. Rather, the reverse is that PFS should be excluded in all women with non-H BPS.
We aim to show that in the female, the PFS is one of the possible aetiologic causes of organ pain, focusing on the urinary bladder. However, distal bowel dysfunction can also be caused by ligament weakness in the small pelvis, thereby extending the PFS symptom complex as summarized diagrammatically in the algorithm rectangle discussed later in this work. Though visceral pain mechanisms are applied to explain CPP causation and cure by USL repair in this work, organ dysfunction might also be explained by disturbances in central nervous system functioning, such as sensitization, cross-organ sensitization, and viscerosomatic reactions caused by emotional stress responses. The pain and other symptom pathogeneses described in this work are limited to PFS-linked experimental data.
Different Anatomical Pathways of PFS
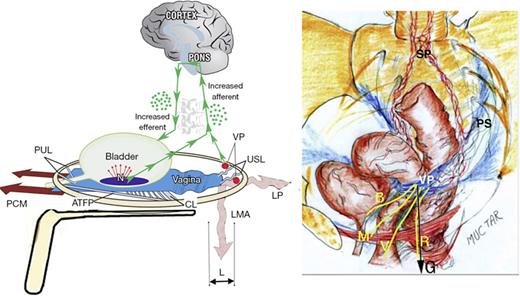
Though the component symptoms of PFS are considered to be primarily caused by uterosacral (USL) laxity [5], their very different anatomical pathways will be demonstrated later in this work. In brief, urge, frequency, and nocturia were consistent with a urodynamically validated prematurely activated micturition reflex [8] caused by anatomical defects in a binary feedback system [9]. CPP follows a very different pathophysiologic pathway from the bladder [10], being a consequence of de novo impulses from unsupported visceral plexuses (VPs) by USLs, as shown in Figure 1.
“Simulated operation” relieves pain and urge by supporting USL. Left: 3D view of the PUL and USL attachments to the pelvic brim. A gently inserted speculum mechanically supports lax USLs and pelvic visceral nerve plexuses (VP). The test, if successful, decreases afferent pain and urge impulses; the patient reports lessening pain in multiple sites, for example, “B,” “R,” and “M” (right image). Co-occurring urge is also often relieved by speculum support of urothelial stretch receptors “N.” Right: 3D view of pelvic organs. The VP comprises sympathetic plexus (SP) and parasympathetic plexus (PS). The yellow lines represent visceral nerves to and from the end organs. M, muscles; V, vagina/vulva; B, bladder; R, rectum; G, force of gravity acting on “VPs” (left figure); PCM, pubococcygeus muscle; PUL, pubourethral ligament; ATFP, arcus tendineus fascia pelvis; USL, uterosacral ligament; CL, cardinal ligament; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; L, USL laxity.
“Simulated operation” relieves pain and urge by supporting USL. Left: 3D view of the PUL and USL attachments to the pelvic brim. A gently inserted speculum mechanically supports lax USLs and pelvic visceral nerve plexuses (VP). The test, if successful, decreases afferent pain and urge impulses; the patient reports lessening pain in multiple sites, for example, “B,” “R,” and “M” (right image). Co-occurring urge is also often relieved by speculum support of urothelial stretch receptors “N.” Right: 3D view of pelvic organs. The VP comprises sympathetic plexus (SP) and parasympathetic plexus (PS). The yellow lines represent visceral nerves to and from the end organs. M, muscles; V, vagina/vulva; B, bladder; R, rectum; G, force of gravity acting on “VPs” (left figure); PCM, pubococcygeus muscle; PUL, pubourethral ligament; ATFP, arcus tendineus fascia pelvis; USL, uterosacral ligament; CL, cardinal ligament; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; L, USL laxity.
Serendipitous Cure of HL IC/BPS with PFS Protocols
The link between PFS and IC/BPS emerged serendipitously in 2021 with a histologically validated surgical cure for IC/BPS HL [6]. A 73-year-old woman presented with urgency, half-hourly frequency, and nocturia twice per night. She was treated strictly according to the protocols of the PFS. She had laxity of the cardinal and uterosacral ligaments and pubocervical/rectovaginal fascia. Before undergoing surgery, she was tested with a “simulated operation” [11], mechanical support of the vaginal apex (and USLs) with a speculum (Fig. 1), and a 3 × 6 cm cylindrical pessary. Both led to almost complete relief of urgency and pain.
On cystoscopy under general anaesthesia, a bladder capacity of 300 mL, showed diffuse distension bleeding, chronic cystitis with patchy mucosal reddening, glomerulations, and a non-ulcerating HL [6]. Resection biopsies showed large numbers of mast cells within the muscular layer. Postoperatively, there was a good anatomical correction of the apical descensus. The patient reported that her symptoms were gradually disappearing, with voiding reduced to 5–6 times daily and nocturia once. A follow-up cystoscopy showed complete healing of the HL, no bleeding, and no glomerulations. There was a substantial cure for the prolapse and PFS symptoms at the 3 years review.
Dr. Scheffler raised the question, “As PFS symptoms are identical with those of interstitial cystitis (IC) as defined [1], are PFS and IC similar conditions? If so, then patients with IC who have a positive speculum test are, at least theoretically, potentially curable by USL repair. These questions need to be explored” [3].
Surgical Testing of the PFS and IC/BPS Equivalence Hypothesis
Goeschen et al. [12] tested Scheffler’s hypothesis that IC/BPS and PFS were substantially equivalent conditions for truth or falsity. A retrospective study was performed with 198 women who presented with CPP, uterine/apical prolapse of varying degrees, and PFS symptoms. All 198 women had been treated by posterior USL sling repair. Goeschen et al. used the same PFS diagnostic protocols as Scheffler, a validated questionnaire: “simulated operations” (mechanically supporting USLs with a vaginal speculum test to test for relief of urge and pain (Fig. 1) [11], urodynamics, and also transperineal ultrasound.
The 198 patients who had presented with CPP had 313 urinary symptoms, which conformed to the definition of IC/BPS [12]. However, there were no HLs or ulcers found on cystoscopy. The cure rate after USL sling repair was CPP 74%; urge incontinence 80%; frequency 79.6%; abnormal emptying 53%; nocturia 79%; obstructive defecation 80%. Goeschen et al. findings supported the hypothesis that non-Hunner’s IC and PFS may be similar conditions. Retrospective examination of data from women who had undergone USL slings based on PFS protocols by Liedl et al. [13, 14], Inoue et al. [15, 16] Petros, Bornstein [17, 18], Himmler et al. [19‒21] gave similar cure rates for CPP and individual bladder symptoms to Goeschen et al. These studies collectively validated the USL cause-effect relationship hypothesised by Scheffler for PFS and non-H IC/BPS. There were no reports of Hunner’s ulcers or lesions during intraoperative cystoscopy in any of these studies.
CPP USL Aetiopathogenesis
The first PFS symptom which we analyse is CPP “of unknown origin” (Fig. 1). CPP is by far the most disturbing PFS symptom. Its diagnosis and cure by USL plication was described for the first time in the English literature in 1996 by Petros [10]. In a laparoscopically controlled study of 28 women who had native USL surgery (6 nulliparas), Petros found no abdominal cause for the CPP. The relief of pain when it occurred was immediate, 85% at 6 weeks and 70% at 12 months. A feature of the pain was wide variation in intensity, sufficiently severe for 6 of the women to be admitted into hospital as emergencies [10]. A ring pessary inserted preoperatively improved the CPP in half of the women [10]. Following USL native ligament plication [10], multiple sites of CPP were cured, which led to the hypothesis that the cause of the CPP was laxity in the visceral pelvic plexuses (VP), T11-L2 [10], which generated de novo afferent impulses to the brain, which were interpreted as pain from the end organs (see short video link for pathogenesis of CPP and urge: https://youtu.be/g3SXKzD4it8?si=DwTW2BTm58pOqzzz).
Association of PFS and Vulvodynia
In 2003, Petros and Bornstein [18] together prospectively assessed three women aged 45, 45, and 47 years, parity 3–4, with CPP, bladder symptoms, and also vulvodynia. All 3 had great difficulty having sexual intercourse and had undergone either Fenton’s operation or vaginal stretching. USL sling surgical repair of USLs was performed. All were cured or improved of both their bladder, pain, and vulvodynia symptoms on postoperative review. These were the first cases in the literature, which demonstrated the link between PFS vulvodymia and non H IC/BPS, which were cured by USL repair. It was these 3 cases, which inspired the Bornstein local anaesthetic test applied to the VPs.
Bornstein Test for VP Origins of CPP
The question arose about testing the hypothesis that CPP and vulvodynia were also caused by unsupported VPs. The hypothesis was tested by injection of local anaesthetic in the position of the USLs [2] in 11 patients [22]. The pain was relieved for 20 min on both sides in 9 patients and on 1 side in 2 women.
Bornstein Test for Relief of Pain in Women with IC/BPS
Overall, 5 mL of xylocaine 1% local anaesthetic solution were injected into the position of the USLs of 3 women with known painful bladder syndrome/IC. All had vascular glomerular changes in the bladder wall vessels observed during cystoscopy and bladder distension. Their symptoms included vulvodynia, low abdominal pain, dyspareunia, nocturia, urgency, and abnormal bladder emptying. The abdominal, urethral, introital, and cervical tenderness and pain demonstrated objectively in all 3 patients immediately before the intervention disappeared entirely or substantially improved within 5 min of the injection [23].
Binary Model for Normal Bladder Function
Bladder symptoms form the second limb of IC/BPS symptoms. The purpose of this section is to explain the anatomical pathways responsible for IC/BPS bladder function and dysfunction [9, 24]. Normal bladder function (as shown in Fig. 2) is cortically controlled and operates in only two states, either closed (most of the time) or open (when the bladder requires emptying) [9, 24]. Binary control functions like an electric switch, toggling between two reflexes: urethral closure or opening (micturition). In the original experiment, the handwashing test blocked the closure reflex (white arrows, Fig. 2), allowing the micturition reflex to dominate [9].
Binary model for bladder control by 2 opposing reflexes, either closure or micturition. Schematic 3D sagittal view, system in normal closed mode. PCM, pubococcygeus muscle; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; PUL, pubourethral ligament; USL, uterosacral ligament; N, urothelial stretch receptors; CX, cervix; CL, cardinal ligament; ATFP, arcus tendineus fascia pelvis; small green arrows, afferent and efferent nerves; white arrows, cortical suppression. Control of SUI: PCM closes the distal urethra from behind; LP/LMA stretches the proximal urethra around PUL to close the bladder neck. Control of urge: afferent impulses “X” from stretch receptors “N” signal bladder fullness reflexly suppressed cortically (white arrows) or peripherally by a musculo‐elastic mechanism, vaginal stretching by 3 striated pelvic muscles contracting against PUL and USL. Micturition: the closure reflex is shut down and the micturition reflex is activated. PCM relaxes. LP and LMA open out the posterior wall of the urethra (white broken lines below the urethra). Dysfunction: any lesion in the circuit can cause retention or loss of urge control: cortex; collagen loss in PUL, USL weakens pelvic muscle contractile strength; excitation of “N” by inflammation, tumour; MS in afferent nerves (retention), MS in efferent nerves (leakage).
Binary model for bladder control by 2 opposing reflexes, either closure or micturition. Schematic 3D sagittal view, system in normal closed mode. PCM, pubococcygeus muscle; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; PUL, pubourethral ligament; USL, uterosacral ligament; N, urothelial stretch receptors; CX, cervix; CL, cardinal ligament; ATFP, arcus tendineus fascia pelvis; small green arrows, afferent and efferent nerves; white arrows, cortical suppression. Control of SUI: PCM closes the distal urethra from behind; LP/LMA stretches the proximal urethra around PUL to close the bladder neck. Control of urge: afferent impulses “X” from stretch receptors “N” signal bladder fullness reflexly suppressed cortically (white arrows) or peripherally by a musculo‐elastic mechanism, vaginal stretching by 3 striated pelvic muscles contracting against PUL and USL. Micturition: the closure reflex is shut down and the micturition reflex is activated. PCM relaxes. LP and LMA open out the posterior wall of the urethra (white broken lines below the urethra). Dysfunction: any lesion in the circuit can cause retention or loss of urge control: cortex; collagen loss in PUL, USL weakens pelvic muscle contractile strength; excitation of “N” by inflammation, tumour; MS in afferent nerves (retention), MS in efferent nerves (leakage).
Control of Urgency in the Normal Woman
For urgency to be controlled in the normal woman, there must be sufficient tension in the vagina to support the urothelial stretch receptors “N” from below (visible in Fig. 2). This tension is created by the opposite contractions of the pubococcygeus muscle (PCM) stretching the vagina forwards, the levator plate (LP) stretching the vagina backwards, and conjoint longitudinal muscle of the anus (LMA) contracting downwards. This reflex action prevents “N” from firing off the afferent emptying impulses, which activate the micturition reflex to empty, felt as “an urge to go” by the patient [24].
Urethral Closure on Effort in the Normal Woman
The same three directional forces close the urethra distally and at the bladder neck (as seen in Fig. 2). Distally, PCM pulls the distal vagina forward against the PUL to close the urethra from behind. At the bladder neck, LP stretches the proximal vagina and urethra backward against the PUL to tighten them; LMA contracts down against the USL to rotate the now tensioned bladder base around the arc of Gilvernet to close the urethra at the bladder neck (see video of three directional muscles: https://youtu.be/GkK5V6LvC3w?si=sWj_6Pk9xpseoSnI).
Micturition in the Normal Woman
With reference to Figure 2, PCM relaxes; LP/LMA pulls down the anterior part of LP to open the posterior wall of the urethra (white broken lines). This external opening mechanism exponentially reduces resistance to urine flow from the contracting detrusor following Poiseuille’s law, where flow is inversely proportional to the fourth power of the radius. The detrusor smooth muscles contract collectively in a coordinated manner, similar to a spasm, to empty (see micturition video: https://youtu.be/nK0CQmaS-5E?si=fPZyZC6AL8sYpFcS).
Pathogenesis within the Binary Model
Any abnormality in the binary control circuit can affect the micturition reflex, leading to retention or OAB, as seen in Figure 2. Neurological lesions such as stroke (cortex), spinal cord injury, and multiple sclerosis in the afferent circuit can cause retention. Similarly, multiple sclerosis in the efferent nerve circuits can affect peripheral control, resulting in urgency.
Lax PUL and USL ligaments weaken the three directional muscles that contract against them, diminishing all three muscle functions. Critically, these weakened functions often co-occur, manifesting as closure (stress urinary incontinence [SUI], urine leakage), evacuation difficulties, retention, and the urge control mechanism (urge, frequency, nocturia).
Local lesions such as inflammation, urothelial cancer, and cervical fibroid pressure can stimulate “N” to increase afferent impulses, which the brain perceives as urgency. If these afferent impulses from “N” cannot be suppressed directly or by activation of the peripheral musculoelastic mechanism (visible in Fig. 2), the micturition reflex is activated, causing OAB and incontinence.
Urge as a Prematurely Activated Uncontrolled Micturition
An urodynamically controlled experiment demonstrated that what was then known as “detrusor instability” is equivalent to a prematurely activated micturition [8]. The pattern of urine loss was identical to that seen by Tanagho during normal micturition [25]: (i) sensation of urge; (ii) fall in proximal urethral pressure; (iii) rise in detrusor pressure; (iv) urine loss (see also https://doi.org/10.1002/nau.24990).
OAB (urge, frequency, nocturia). Weakness in either the PUL or USL impairs the muscles contracting against them, as seen in Figure 3. With reference to Figure 2, weakened PCM and LP/LMA muscles cannot adequately stretch the vagina to support “N,” leading to afferent emptying impulses reaching the cortex, interpreted as an “urge to go” by the patient [24]. Beyond a critical point, the micturition reflex temporarily overrides control. The system becomes unstable and swings between “open” and “closed,” which is the key characteristic of OAB and urodynamic “detrusor overactivity”.
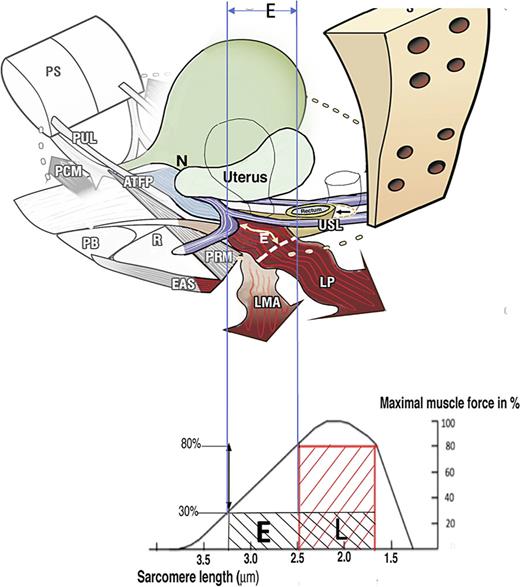
How ligament laxity diminishes striated muscle force? Upper image: Anatomy. If USLs are overstretched by length “E,” the uterus prolapses, LP and LMA lengthen by “E.” The wavy shape of LP and LMA indicate diminution of contractile strength. Lower image: Sarcomere (striated muscle fibres). A striated muscle contracts optimally of a short length only (L). Elongation of the muscle to “E” diminishes contractile force. USL, uterosacral ligament; LP, levator plate; LMA, conjoint longitudinal muscle of the anus.
How ligament laxity diminishes striated muscle force? Upper image: Anatomy. If USLs are overstretched by length “E,” the uterus prolapses, LP and LMA lengthen by “E.” The wavy shape of LP and LMA indicate diminution of contractile strength. Lower image: Sarcomere (striated muscle fibres). A striated muscle contracts optimally of a short length only (L). Elongation of the muscle to “E” diminishes contractile force. USL, uterosacral ligament; LP, levator plate; LMA, conjoint longitudinal muscle of the anus.
Emptying: Urinary Retention
Weak USLs reduce the contractile force of the LP and LMA muscles that contract against them, as seen in Figure 3. The weakened LP/LMA cannot open the urethra before micturition. Consequently, the detrusor contracts against a relatively unopened urethra against high urethral resistance, which the cortex correctly interprets as “obstruction”.
Anatomical Pathway of Nocturia
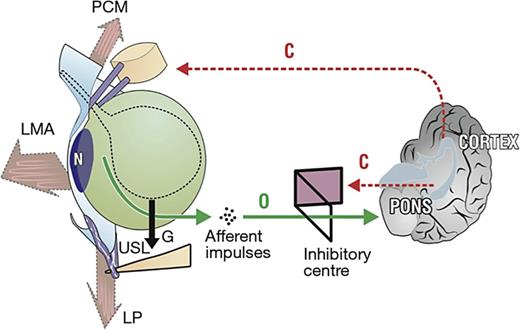
At night, the woman is in the supine position, so the muscle vectors cannot stretch the vagina to support the stretch receptors “N” (Fig. 4). A loose USL cannot prevent the proximal vagina and bladder base from being pulled down by gravity “G” (Fig. 4). The downward stretching simulates the stretch receptors “N” activating the micturition reflex, which the cortex interprets as “urge.” A pessary or tampon can often alleviate nocturia if it can sufficiently support the weakened USLs.
The anatomy of nocturia pelvic muscles (large arrows) is relaxed. As the bladder (broken outline) fills, it is distended downwards by gravity “G.” If the uterosacral ligaments (USLs) are weak, they continue to descend until the stretch receptors “N” are stimulated, activating the micturition reflex “O” once the cortical closure reflex “C” has been overcome. PCM, pubococcygeus muscle; LP, levator plate; LMA, conjoint longitudinal muscle of the anus.
The anatomy of nocturia pelvic muscles (large arrows) is relaxed. As the bladder (broken outline) fills, it is distended downwards by gravity “G.” If the uterosacral ligaments (USLs) are weak, they continue to descend until the stretch receptors “N” are stimulated, activating the micturition reflex “O” once the cortical closure reflex “C” has been overcome. PCM, pubococcygeus muscle; LP, levator plate; LMA, conjoint longitudinal muscle of the anus.
Non-Surgical Treatment by Squatting-Based Pelvic Floor Exercises
In a retrospective study, two separate groups comprising 138 women performed squatting-based exercises for SUI, OAB, pain, and emptying symptoms [26]. All participants completed validated pelvic questionnaires [27], pad tests, and diaries.
Across both groups of mainly premenopausal women, 61%–80% of the women had greater than 50% improvement in symptoms of SUI, urge, frequency, nocturia, abnormal emptying, and post-void residual urine at a 3-month review; 37 of the 138 women from both studies fulfilled the ICS definition of IC, CPP, and co-occurrence of one bladder symptom, which are identical or similar symptoms as those characterizing PFS. The anatomic rationale for the cure was that the squatting-based exercises strengthen the three reflex muscles controlling bladder functions and the USLs against which these muscles contract [26].
Dr. Skilling concluded that whether the conditions IC and PFS are identical or distinct, their component symptoms can, in the main, be improved by more than 50% by a squatting-based exercise regime.
Validated Questionnaire Applied to Known IC Women
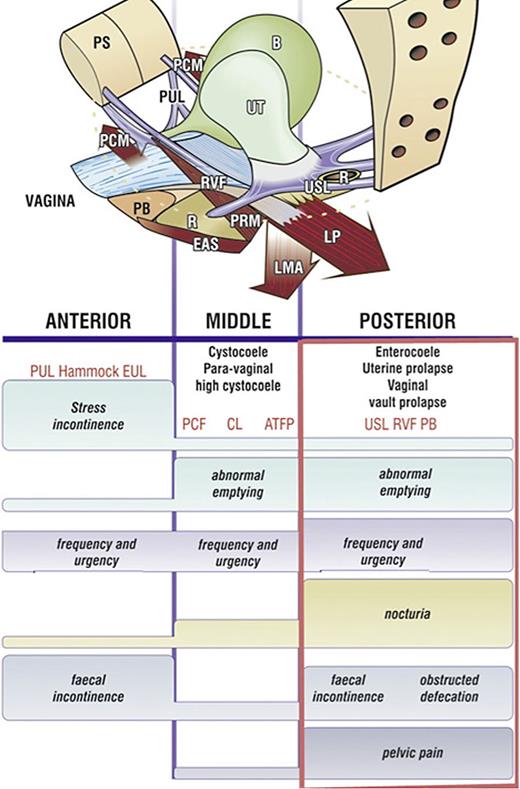
Sandy McNicol, the secretary of the Canadian Interstitial Cystitis Society, distributed the validated structured Integral Theory Questionnaire to members of the IC society. Of 136 questionnaires completed in 2000, 130 had two or more parameters indicative of posterior ligamentous laxity (visible in rectangle, Fig. 5) [28]. Urgency and pelvic pain are key symptoms in patients with PFS.
Pictorial diagnostic algorithm. Symptoms indicate which ligaments are damaged. Tick every box where a symptom occurs, and the diagnosis of ligament and damage-associated prolapse appears. The rectangle indicates the symptoms associated with USL laxity. The height of the bar indicates the probability of association of a symptom with a particular zone. The connective tissue structures causing prolapse and pelvic symptoms fall naturally into 3 zones. Anterior zone: external meatus to bladder neck. Middle zone: bladder neck to anterior cervical ring. Posterior zone: posterior cervical ring to PB. CPP and nocturia are uniquely caused by USL laxity, stress urinary incontinence by PUL, pubourethral ligament; hammock, suburethral vagina. EUL, external urethral ligament; CL, cardinal ligament; PCF, pubocervical fascia; ATFP, arcus tendinous fascia pelvis; PB, perineal body; RVF, rectovaginal fascia; USL, uterosacral ligament.
Pictorial diagnostic algorithm. Symptoms indicate which ligaments are damaged. Tick every box where a symptom occurs, and the diagnosis of ligament and damage-associated prolapse appears. The rectangle indicates the symptoms associated with USL laxity. The height of the bar indicates the probability of association of a symptom with a particular zone. The connective tissue structures causing prolapse and pelvic symptoms fall naturally into 3 zones. Anterior zone: external meatus to bladder neck. Middle zone: bladder neck to anterior cervical ring. Posterior zone: posterior cervical ring to PB. CPP and nocturia are uniquely caused by USL laxity, stress urinary incontinence by PUL, pubourethral ligament; hammock, suburethral vagina. EUL, external urethral ligament; CL, cardinal ligament; PCF, pubocervical fascia; ATFP, arcus tendinous fascia pelvis; PB, perineal body; RVF, rectovaginal fascia; USL, uterosacral ligament.
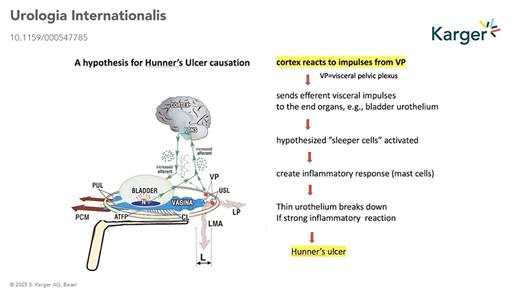
Hypothetical Pathways of HL and Hunner’s Ulcer IC/BPS
In his 1996 paper, based on end-organ pain sites relieved by USL plication: (lower abdomen, cervical excitation pain, deep dyspareunia, coccyx, introitus) Petros hypothesised that nerve impulses from unsupported VPs were causing the pain. One hypothesis to explain HL and Hunner’s ulcer is that once afferent signals from the VPs are registered as pain by the brain, efferent signals are sent via the visceral nerve system, eliciting an inflammatory response at the end organ (visible in Fig. 1). A second, more recent hypothesis proposes a similar mechanism but involves the axon reflex. According to this hypothesis, such conditions involve axon reflexes. Stimuli such as gravity applied to unsupported nerve branches within the visceral pelvic plexus trigger centrally propagating impulses, which then progress antidromally. These impulses influence innervated tissues through cytokine release and nociceptor stimulation, perpetuating inflammatory processes at the end organs and pain perception.
Both hypotheses raise the question, “are IC/BPS, vulvodynia, Posterior Fornix Syndrome, other pain sites, all different phenotypes of the CPP syndrome”? If so, the hypothesis opens several new research directions and predicts inflammatory findings on histological examination of tender end-organ pain sites.
Conclusions
The PFS is a new direction for IC/BPS. High rates of cure have been reported in hundreds of women following USL repair in women with PFS [12‒17]. We emphasize, and it is evident in the text of our paper, that we are not claiming that PFS is the cause of all non-H IC/BPS. Rather, the reverse: that PFS should be excluded in all women with non-H BPS.
If women diagnosed as non Hunner’s IC/BPS fit the diagnostic criteria of PFS, their condition can be considered similar to PFS. As such, it is potentially curable by USL repair. All of us have seen women who do not fit these criteria, and we do not operate on such women.
Obstacles to answering the question of whether PFS and ICS/BPS in women are one and the same condition are few. Speculum or vaginal probe tests can easily be performed as part of the initial IC/BPS examination. The Bornstein local anaesthetic test in the distal USLs can definitively confirm VP causation of the pain component. Meanwhile, IC/BPS experts with gynaecological skills can perform USL plication in women with uterine prolapse and PFS symptoms using the transverse vaginal incision technique described, preferably employing wide-bore collagenopoietic No. 2 polyester sutures for longer term results [29] (see video link for summary of aetiopathogenesis and actual surgical technique: https://youtu.be/6LAqp9rfDDU?si=K9AUrrAscQy6Sh73).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Content: M.W., B.L., K.G., J.B., and P.P. Conceptualization, writing: M.W., B.L., and P.P. Reviewing, checking manuscript, and additions: B.L., P.P., K.G., and J.B. Figures and videos: P.P.
Footnotes
Predictably co-occurring symptoms of chronic pelvic pain, urgency, frequency, nocturia, abnormal emptying, caused by USL laxity, and cured/improved by USL repair.
The most quoted example of this principle was the discovery of black swans in Western Australia in 1697. In January 1697, Dutch explorer Willem de Vlamingh and his crew were the first Europeans to document the existence of black swans in what is now Western Australia. They observed these birds in abundance along the Swan River, which they subsequently named after the species. This discovery was significant because, prior to this, Europeans had only known of white swans, and the existence of black swans challenged their long-held assumptions about the species.