Abstract
Introduction: Abiraterone acetate is an inhibitor of androgen biosynthesis and is approved as a treatment for metastatic castration-resistant and metastatic castration-sensitive prostate cancer. Neither relugolix nor abiraterone is known to cause acute kidney injury (AKI). Case Presentation: A 72-year-old Caucasian man with metastatic prostate cancer presented with non-oliguric severe AKI 1 week after receiving simultaneous therapy with relugolix and abiraterone. The patient had been on abiraterone for 3 weeks. His physical examination was unremarkable. Blood work on admission revealed hypocalcemia, and elevated creatinine at 3.6 mg/dL. A kidney biopsy confirmed the diagnosis of a high-grade subacute tubular damage, most likely due to nephrotoxicity. The patient did not respond to intravenous isotonic fluids, the discontinuation of relugolix, abiraterone, and rosuvastatin, as well as to hemodialysis. He died 22 days after hospital admission. Conclusion: We report the first case of biopsy-proven drug-induced AKI in a cancer patient acutely exposed to relugolix and abiraterone. Whether one of these drugs individually, or their combination, was the cause of the AKI is unknown. Nonetheless, our report is hypothesis-generating for further investigations on the effect of these drugs.
Introduction
Abiraterone acetate (hereafter referred to as abiraterone) is a selective and irreversible inhibitor of CYP17 that blocks the synthesis of androgens in tissues such as the prostate, tumor cells, adrenal glands and testes [1]. In 2011, abiraterone, in combination with prednisone, was initially approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC) in patients previously treated with chemotherapy. Furthermore, in 2013, abiraterone was approved by regulatory agencies in the SA and Europe for chemotherapy-naive mCRPC [2]. It has been shown to significantly increase overall survival and provide additional clinical benefits in patients with mCRPC who have not received chemotherapy and in those who have received docetaxel. More recently, the addition of abiraterone plus prednisone to androgen deprivation therapy was also found to be associated with longer overall survival and longer radiographic progression-free survival than androgen-deprivation therapy alone in patients with newly diagnosed, metastatic castration-sensitive prostate cancer [1].
The gonadotropin-releasing hormone (GnRH) analog drug class consists of both GnRH agonists and antagonists and is primarily used for androgen-deprivation therapy in the treatment of prostate cancer [3]. Relugolix is the first and only Food and Drug Administration (FDA)-approved oral GnRH antagonist for the treatment of adult patients with advanced prostate cancer. Unlike GnRH agonists, relugolix decreases serum testosterone levels without testosterone flares at treatment initiation [4]. Eliminating testosterone flares increases the tolerability of this medication by reducing side effects such as ostealgia and urinary retention. Relugolix also has a lower relative risk of cardiovascular events such as myocardial infarction and stroke when compared to GnRH agonists. Additionally, other GnRH analogs including leuprolide (GnRH agonist) and degarelix (GnRH antagonist) are administered subcutaneously or intramuscularly, and therefore carry a risk of injection site reaction [4]. The oral administration of relugolix eliminates this risk, making it a favorable option for patients. Aside from an injection site reaction, the only other known skin reaction related to GnRH analogs is skin reddening during acute hot flashes. Relugolix, compared to leuprolide, has shorter time to testosterone suppression, higher rate of sustained testosterone suppression, and faster testosterone recovery upon therapy completion [4, 5].
Hereby, we report a case of AKI induced by the combination of relugolix and abiraterone. A possible cause is discussed in more detail in the discussion.
Case Report
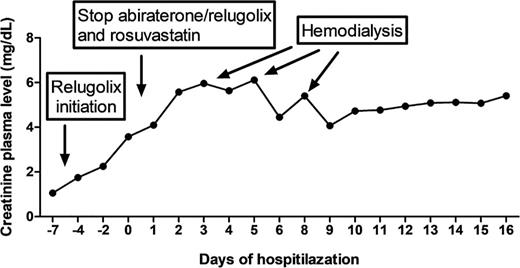
A 72-year-old Caucasian man with mCRPC on abiraterone and relugolix was admitted to the Emergency Department with complaints of weakness, fever, and progressive fatigue for several days. At the time of presentation, his creatinine was notably elevated to 3.6 mg/dL. The patient had a history of coronary artery disease, atrial fibrillation, status post placement of drug eluding stents, hyperlipidemia, osteopenia, and gastroesophageal reflux disease. His prostate cancer was diagnosed 9 years ago (in 2016), and at this time, he received radical prostatectomy. Six years later (in 2022), he developed local recurrence and subsequently experienced progression of disease while on leuprolide with enzalutamide, despite undergoing definitive external beam radiotherapy, five cycles of docetaxel, six cycles of cabazitaxel, and two cycles (177)Lu-PSMA radioligand therapy. He started with abiraterone 1,000 mg once daily 3 weeks as well as 1 week with relugolix 120 mg daily before presentation. His other medications included bisoprolol 10 mg daily, pantoprazole 20 mg daily, edoxaban 60 mg daily (paused, prior due to gross hematuria), pregabalin 150 mg twice daily, rosuvastatin 40 mg daily, and hydromorphone 20 mg daily. Physical examination in the emergency room was unremarkable. Blood tests highlighted an acute renal failure with serum creatinine level of 3.6 mg/dL (normal value (NV): 0.7–1.2 mg/dL; last value measured at 2.3 mg/dL 2 days earlier and a value of 1.06 mg/dL before starting relugolix), urea level of 49 mg/dL (NV: 8–23 mg/dL), and no evidence of rhabdomyolysis with CK level of 192 U/L (NV: 20–200 IU/L) and normal potassium and lactate dehydrogenase levels. Urinalysis revealed neither nitrites nor leukocyte esterase. Upon admission to the hospital, rosuvastatin, abiraterone, and relugolix were discontinued and the other drugs adapted to the reduced kidney function. The diagnosis of drug-induced AKI was suspected. He received aggressive hydration without improvement in creatinine levels, and due to his lack of response to fluid resuscitation and missing improvement in kidney function (shown in Fig. 1), three sessions of hemodialysis and a diagnostic renal biopsy were performed. This showed a high-grade subacute tubular damage, most likely due to nephrotoxicity. As the prognosis was very poor, best supportive care was provided and the patient died 22 days after hospital admission.
Discussion
In clinical trials of abiraterone, the most common adverse events of any grade (≥10%) were fatigue, arthralgia, hypertension, nausea, edema, hot flush, diarrhea, vomiting, upper respiratory infection, cough and headache. The most common laboratory abnormalities of any grade (>20%) were anemia, elevated alkaline phosphatase, hypertriglyceridemia, lymphopenia, hypercholesterolemia, hyperglycemia, and hypokalemia [1].
Few cases of abiraterone-induced rhabdomyolysis with AKI have been described since 2011. Interestingly abiraterone was associated with rosuvastatin in nearly all the cases previously reported [1]. Our patient was 72 years old, had a normal kidney function, and had been treated with high doses of rosuvastatin for a few years, potential risk factors for (rhabdomyolysis induced) AKI [1, 6]. However, he had never experienced rhabdomyolysis before, nor was his creatine kinase elevated. Based on temporal concordance (initiation of abiraterone and relugolix a few weeks before), we hypothesized that the interaction between abiraterone and relugolix (with rosuvastatin) was the cause of AKI in our patient [1]. Rosuvastatin undergoes hepatic metabolism, with a low conversion into N-desmethyl rosuvastatin through CYP2C9, 90% of the drug being eliminated in the bile without transformation. However, hepatic metabolism and elimination depend on the extraction of the drug from the portal blood by the liver, through uptake transporters. Rosuvastatin is the substrate of the uptake transporter organic anion transporting polyprotein 1B1 (OATP1B1) and of the efflux transporter breast cancer resistance protein (BCRP) [1]. Abiraterone is known to act only on CYP17, with no apparent interference with statin metabolism. According to the product information, abiraterone metabolites inhibit the hepatic uptake transporter OATP1B1 in vitro, which may theoretically affect OATP1B1-mediated uptake of rosuvastatin, increase its levels, and therefore predispose to toxicity [7]. To our knowledge, there are no reported cases of AKI with either acute or chronic exposure to abiraterone, alone or in conjunction with rosuvastatin [1]. It is also known from the product information that relugolix is an inhibitor of BCRP in vitro.
The clinical importance of OATP1B1 in the pharmacokinetics of drugs has been suggested by several studies that focused on the effect of commonly occurring single-nucleotide polymorphisms in OATP1B1 [1]. The OATP1B1*15 variant, found in 16–24% of the population in Europe and America, is associated with a reduced transport activity and increased plasma levels of certain OATP1B1 substrates. Moreover, the administration of other OATP1B1 inhibitors such as cyclosporine, gemfibrozil, cobicistat, and several antidiabetic drugs (glimepiride, pioglitazone) has been shown to increase the serum concentrations of rosuvastatin, with clinically significant toxicity in some cases [1].
We report the first case of AKI in association with acute exposure to relugolix and abiraterone. Based on our case, we are unable to determine whether AKI was caused by one of these two drugs, their mutual interaction, or their interaction with another commonly used drug, rosuvastatin. We did not find any relevant interactions between the patient's medications by using a drug interaction software. Nonetheless, this case exposes the need to carefully monitor patients exposed to relugolix and abiraterone to recognize potential severe adverse effects associated with these therapies [7]. We suggest a new interaction mechanism leading to AKI, through an abiraterone and/or relugolix-induced inhibition of the plasmatic uptake of rosuvastatin by OATP1B1, leading to an increased plasmatic concentration of rosuvastatin and a higher risk of nephrotoxicity. Therefore, we suggest that the association of rosuvastatin and abiraterone should be avoided [1]. In conclusion, we recommend monitoring for early signs of AKI in high-risk patients exposed to either relugolix or abiraterone, particularly if other identifiable risk factors are present, as seen in our patient (e.g., advanced age, for coronary artery disease, and statin use).
Statement of Ethics
Written informed consent was obtained from the patient for the publication of this case report before death. Ethical approval is not required for this study in accordance with local guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Moustafa Elleisy: writing – original draft and designed figures. Irene Resch, Ozan Yurdakul, Nicolai Hübner, and Shahrokh F. Shariat: review and editing.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.