Abstract
Introduction: This study aims to evaluate the effectiveness of prostatic artery embolization (PAE) in treating benign prostatic hyperplasia (BPH), focusing on identifying the predictors of treatment failure and assessing patient satisfaction and second-line therapies for patients who undergo reoperation. Methods: We conducted a monocentric, retrospective study involving 344 patients who underwent PAE from 2017 to 2022. The minimum follow-up time was 12 months. Baseline data were retrospectively collected. A single follow-up questionnaire was administered at the time of the study. Included patients were ≥50 years, with a prostate volume ≥40 mL, an International Prostate Symptom Score (IPSS) ≥8 and were unresponsive to medical therapy. Results: Among 156 participants, the reoperation rate at 5 years was 28.2%. Baseline IPSS and post-void residual volumes (PVR) were significant predictors of therapy failure. Higher satisfaction was associated with younger age (p = 0.01), larger prostate volume (p = 0.02), and lower PVR (p = 0.03). Patients with higher satisfaction had better reoperation-free rates at 60 months (p = 0.002). Conclusions: PAE is effective in reducing symptoms in patients with BPH; however, the reoperation rate emphasizes the importance of careful patient selection. Study limitations include potential selection bias, missing data, the single-center setting, and the use of a single follow-up questionnaire.
Introduction
Benign prostatic hyperplasia (BPH) significantly impacts the quality of life in aging men by causing bothersome lower urinary tract symptoms. While not every case of prostatic enlargement is clinically significant, the probability of experiencing benign prostatic obstruction along with lower urinary tract symptoms increases with age. Research shows that 56% of men younger than 80 years and 70% of those older than 80 years report symptoms typical of BPH [1].
Until the early 2000s, transurethral resection of the prostate (TURP) was considered the gold standard of BPH treatment [2]. Although highly effective, TURP is associated with certain complications such as retrograde ejaculation (RE) and incontinence [3], prompting the exploration of safer alternatives. Among these, prostatic artery embolization (PAE) has emerged as a promising option for patients seeking an effective yet less invasive alternative [4, 5]. Performed under local anesthesia, PAE selectively embolizes the prostatic arteries, leading to ischemic shrinkage of the prostate and progressive symptom relief [6].
Extensive research corroborates PAEs ability to not only improve these symptoms but also to maintain sexual and urinary functions [7, 8]. PAE leads to significant reductions in International Prostate Symptom Score (IPSS) by 5–8 points and a nearly 30% decrease in prostate volume within 6–12 months post-procedure, resulting in measurable improvements in overall symptom relief and quality of life [9, 10].
However, while PAE offers several advantages, it may not always provide the same degree of symptom relief as other minimally invasive treatments and is associated with higher reported reoperation rates [11, 12]. The variability in patient outcomes after PAE underscores the need for better patient selection criteria to optimize treatment success. While PAE is generally associated with fewer adverse events compared to traditional surgical treatments [13], complications still occur and may affect both short- and long-term outcomes [14].
The current literature offers limited insight into the factors that influence both short- and long-term outcomes, particularly regarding reoperation rates, complications, and success rates of second-line therapies following PAE failure. Factors such as age, prostate size, and post-void residual (PVR) volume can influence both patient satisfaction and reintervention rates. Therefore, our study aims to identify the predictors of treatment failure, analyze the factors influencing patient satisfaction, and examine the reoperation rates and second-line therapy choices following PAE failure. This research fills this gap in knowledge by quantitatively evaluating the frequency of reinterventions and qualitatively exploring how different therapies align with patient expectations and satisfaction after PAE.
Patients and Methods
Patients, Setting, and Study Approval
We conducted a retrospective study involving 344 patients who underwent PAE at our institution between January 2017 and January 2022. The inclusion criteria were men aged ≥50 years with symptomatic (BPH), defined as an IPSS ≥8 and a prostate volume of at least 40 mL, who had failed medical therapy with alpha-1 adrenergic receptor antagonists and/or 5-alpha reductase inhibitors for at least 6 months. Patients were required to have a minimum follow-up of 12 months after PAE. The exclusion criteria included patients with prostate cancer, previous prostate surgeries, or incomplete retrospective medical records. Patients who could not be contacted for follow-up were categorized as lost to follow-up. The patient selection process, including exclusions, is detailed in the flow chart (Fig. 1).
Study enrollment and follow-up. Out of 344 patients who underwent prostatic artery embolization, 265 were eligible for the study. After exclusions, 201 provided consent, but 45 either withdrew or failed to return the questionnaire, resulting in a final sample of 156 participants.
Study enrollment and follow-up. Out of 344 patients who underwent prostatic artery embolization, 265 were eligible for the study. After exclusions, 201 provided consent, but 45 either withdrew or failed to return the questionnaire, resulting in a final sample of 156 participants.
Before PAE, all patients underwent a preoperative evaluation, including a digital rectal examination (DRE) and PSA screening based on European Urological Association guidelines. Patients with abnormal PSA levels or abnormal DRE results underwent prostate biopsy or magnetic resonance imaging. The study received approval from the Ethics Committee of the Medical Association of North Rhine. All participants provided written informed consent after receiving information about the study’s objectives and potential risks and benefits.
Data Collection and Study Design
Baseline demographic and clinical data, including prostate volume, age, PSA, PVR volume, and initial IPSS scores, were retrospectively collected from medical records at the time of the procedure. To assess treatment outcomes, a single follow-up questionnaire was sent at the time of the study, collecting updated IPSS scores, current satisfaction levels, complications, any second-line therapies they may have undergone, and their histological findings. If patients were scheduled for a second intervention, we conducted follow-up at least 1 year after their secondary procedure to collect data on the outcomes of the second intervention. While this introduced a prospective component for this subgroup, it does not change the overall retrospective design of the study.
We used a numeric patient satisfaction scale ranging from 1 (lowest) to 6 (highest), similar to standard numeric rating scales applied to many healthcare research settings. This numeric format is often easier for patients to understand, providing an intuitive and quick method to express satisfaction levels. For analysis, we categorized the scale responses as follows: low satisfaction (1–2), moderate satisfaction (3–4), and high satisfaction (5–6).
In the multivariable analysis, we assessed key clinical factors to determine their influence on patient satisfaction and therapy failure. Therapy failure was defined as the need for additional surgical intervention following PAE due to inadequate symptom relief. The factors included in the analysis were patient age, prostate volume, initial IPSS score, PVR volume, reduction in IPSS, complications, and RE. These variables were selected based on their clinical relevance in previous studies of BPH.
Statistical Analysis
We performed both descriptive and inferential statistical analyses. Continuous variables were presented as means with standard deviations (SDs) for normally distributed data, and as medians with interquartile ranges (IQRs) for non-normally distributed data. Categorical data were summarized as frequencies and percentages.
The patient satisfaction scale (ranging from 1 to 6) was analyzed as a continuous variable, but for interpretative clarity, it was also categorized into three levels: “low,” “moderate,” and “high” satisfaction. Normality of continuous variables was tested using the Shapiro-Wilk test.
For group comparisons, we applied independent-samples t tests or ANOVA for normally distributed data, and the Wilcoxon rank-sum test or Kruskal-Wallis test for non-normally distributed data. Chi-square tests or Fisher’s exact tests were used for categorical variables such as RE and reoperation rates, with Fisher’s exact test applied in cases where sample sizes were small. To adjust for potential confounders, we employed multivariable regression analysis, including age, prostate volume, baseline IPSS score, and PVR volume as covariates. Kaplan-Meier survival curves were used to estimate 5-year reoperation-free survival, stratified by patient satisfaction levels, and the log-rank test was used to compare survival curves across groups. Statistical significance was set at a p value of less than 0.05. All analyses were conducted using R version 4.2.2 and GraphPad Prism version 5.0 (San Diego, CA, USA).
Results
Patient Demographics and Outcome Measures
A total of 156 patients were included in the final analysis. Minimum follow-up time was 12 months, with a median follow-up time of 37 months. The mean (SD) age was 66 (6.9) years, the median [IQR] PSA was 3.7 ng/mL [2.1, 5.9], the median [IQR] prostate volume was 70 mL [54, 91.25], and the median [IQR] PVR volume was 50 mL [20, 110]. Five (3.2%) patients presented with a urinary catheter prior to undergoing PAE. Patient characteristics and outcomes grouped by satisfaction level after PAE are listed in Table 1. Out of the 156 patients, 34.6% experienced complications following PAE, which were classified according to the Clavien-Dindo system. The detailed breakdown of adverse events is presented in Table 2.
Baseline characteristics and outcome measures by satisfaction level after PAE
| . | High satisfaction (n = 56) . | Moderate satisfaction (n = 51) . | Low satisfaction (n = 49) . | p value* . |
|---|---|---|---|---|
| Median age, year (SD) | 64.38 (6.22) | 68.75 (7.05) | 66.69 (7.04) | 0.004 |
| {range} | {51–77} | {56–81} | {51–80} | |
| PV, median (IQR) | 80.50 (61.50,100.00) | 60.00 (50.00, 80.00) | 65.00 (54.00, 85.00) | 0.014 |
| {range} | {40–180} | {38–200} | {36–250} | |
| PVR, median (IQR) | 64.00 (30.00, 127.50) | 50.00 (0.00, 90.00) | 50.00 (22.00, 125.00) | 0.199 |
| PSA, median (IQR) | 4.70 (2.82, 7.13) | 2.80 (1.92, 4.20) | 3.60 (2.04, 6.00) | 0.112 |
| Preoperative Qmax, median (IQR) | 10 (6.5–13) | 7.6 (5.6–11) | 6.8 (5.2–8.9) | 0.085 |
| RE, n (%) | 9 (16.1) | 10 (19.6) | 3 (6.1) | 0.133 |
| Preoperative IPSS, median (IQR) | 23.50 (20.00, 27.25) | 22.00 (17.50, 26.00) | 22.00 (17.00, 28.00) | 0.513 |
| Postoperative IPSS, mean (SD) | 9.18 (5.80) | 15.03 (5.05) | 17.59 (6.77) | <0.001 |
| IPSS reduction, mean (SD) | 13.62 (7.62) | 5.33 (7.40) | 2.81 (7.75) | <0.001 |
| . | High satisfaction (n = 56) . | Moderate satisfaction (n = 51) . | Low satisfaction (n = 49) . | p value* . |
|---|---|---|---|---|
| Median age, year (SD) | 64.38 (6.22) | 68.75 (7.05) | 66.69 (7.04) | 0.004 |
| {range} | {51–77} | {56–81} | {51–80} | |
| PV, median (IQR) | 80.50 (61.50,100.00) | 60.00 (50.00, 80.00) | 65.00 (54.00, 85.00) | 0.014 |
| {range} | {40–180} | {38–200} | {36–250} | |
| PVR, median (IQR) | 64.00 (30.00, 127.50) | 50.00 (0.00, 90.00) | 50.00 (22.00, 125.00) | 0.199 |
| PSA, median (IQR) | 4.70 (2.82, 7.13) | 2.80 (1.92, 4.20) | 3.60 (2.04, 6.00) | 0.112 |
| Preoperative Qmax, median (IQR) | 10 (6.5–13) | 7.6 (5.6–11) | 6.8 (5.2–8.9) | 0.085 |
| RE, n (%) | 9 (16.1) | 10 (19.6) | 3 (6.1) | 0.133 |
| Preoperative IPSS, median (IQR) | 23.50 (20.00, 27.25) | 22.00 (17.50, 26.00) | 22.00 (17.00, 28.00) | 0.513 |
| Postoperative IPSS, mean (SD) | 9.18 (5.80) | 15.03 (5.05) | 17.59 (6.77) | <0.001 |
| IPSS reduction, mean (SD) | 13.62 (7.62) | 5.33 (7.40) | 2.81 (7.75) | <0.001 |
PV, prostate volume in ml; PVR, post-void residual in ml; PSA, prostate-specific antigen in ng/mL; RE, retrograde ejaculation; IPSS, international Prostate Symptom Score; IPSS reduction, difference between preoperative IPSS and postoperative IPSS at least 12 months after PAE; Qmax: maximum urinary flow rate in mL/s.
Frequency and classification of nonserious and serious adverse events following prostatic artery embolization
| Clavien-dindo classification . | Adverse event . | Events (n) . | Patients, n (%) . |
|---|---|---|---|
| Grade I | RE | 22 | 22 (14.10) |
| Mild pain | 9 | 9 (5.77) | |
| Urinary urgency | 5 | 5 (3.21) | |
| Urinary incontinence – urge | 2 | 2 (1.28) | |
| Chronic pelvic pain | 2 | 2 (1.28) | |
| Erectile dysfunction | 1 | 1 (0.64) | |
| Grade II | Urinary retention (requiring catheter) | 4 | 4 (2.56) |
| Urinary tract infection | 3 | 3 (1.92) | |
| Acute prostatitis | 2 | 2 (1.28) | |
| Anal venous thrombosis | 2 | 2 (1.28) | |
| Pain requiring admission | 1 | 1 (0.64) | |
| Grade IVb | Urosepsis (requiring ICU) | 1 | 1 (0.64) |
| Total | 54 | 54 (34.62) |
| Clavien-dindo classification . | Adverse event . | Events (n) . | Patients, n (%) . |
|---|---|---|---|
| Grade I | RE | 22 | 22 (14.10) |
| Mild pain | 9 | 9 (5.77) | |
| Urinary urgency | 5 | 5 (3.21) | |
| Urinary incontinence – urge | 2 | 2 (1.28) | |
| Chronic pelvic pain | 2 | 2 (1.28) | |
| Erectile dysfunction | 1 | 1 (0.64) | |
| Grade II | Urinary retention (requiring catheter) | 4 | 4 (2.56) |
| Urinary tract infection | 3 | 3 (1.92) | |
| Acute prostatitis | 2 | 2 (1.28) | |
| Anal venous thrombosis | 2 | 2 (1.28) | |
| Pain requiring admission | 1 | 1 (0.64) | |
| Grade IVb | Urosepsis (requiring ICU) | 1 | 1 (0.64) |
| Total | 54 | 54 (34.62) |
Bold values represent the total number of adverse events after PAE and their percentage relative to all reported adverse events (100%).
Influential Factors in Patient Satisfaction after PAE
The IPSS decreased significantly by a mean of 8 points. A correlation was noted between the degree of IPSS reduction and the level of patient satisfaction (Pearson’s correlation coefficient r = 0.62, p < 0.001, 95% CI: 0.39–0.65), as depicted in Figure 2. In multivariable linear regression analysis, younger age (p = 0.01, 95% CI: 0.08–0.37), larger prostate volume (p = 0.02, 95% CI: 0.05–0.36), and lower PVR volume (p = 0.03, 95% CI: 0.21–0.48) were independently associated with higher patient satisfaction. Other variables, including the initial IPSS score, baseline PSA level, complications, and the presence of RE post-PAE, did not show a significant correlation with patient satisfaction (p > 0.1). Univariate and multivariable analysis results are provided in online supplementary Table S1 (for all online suppl. material, see https://doi.org/10.1159/000547152).
IPSS reduction at least 12 months after PAE. Illustrates the relationship between the degree of IPSS reduction and patient satisfaction levels following PAE in patients with benign prostatic hyperplasia. Error bars: represent the standard deviation (SD) of the IPSS reduction within each satisfaction category. IPSS, International Prostate Symptom Score; PAE, prostatic artery embolization.
IPSS reduction at least 12 months after PAE. Illustrates the relationship between the degree of IPSS reduction and patient satisfaction levels following PAE in patients with benign prostatic hyperplasia. Error bars: represent the standard deviation (SD) of the IPSS reduction within each satisfaction category. IPSS, International Prostate Symptom Score; PAE, prostatic artery embolization.
Predictors of PAE Failure and Second-Line Therapy Outcomes
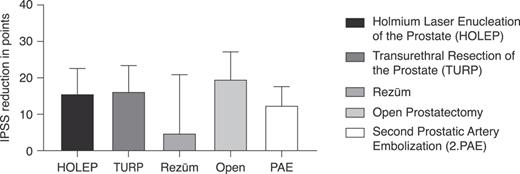
At 5 years post-PAE, the reoperation rate was 28.2%, with 44 patients having undergone a second intervention. Among the 44 patients who underwent a second operation, 20 (45.5%) underwent HOLEP, 13 (29.5%) underwent TURP, 4 (9.1%) underwent a second PAE, 3 (6.8%) underwent Rezūm, 3 (6.8%) underwent simple open prostatectomy, and 1 (2.3%) underwent aquablation. Five patients with pre-procedural urinary catheters required reoperation, but no sensitivity analysis was conducted due to the small sample size. Furthermore, we examined the IPSS score after the second operation, as shown in Figure 3, and noted that ablative interventions showed a significant improvement in the IPSS (p < 0.0001; 95% CI: 12.75, 18.74). Multivariable regression analysis identified significant predictors of treatment failure. Patients with higher initial IPSS scores (p < 0.01) and those with elevated PVR volumes (p = 0.03) were more likely to undergo reoperation.
IPSS reduction at least 12 months after the second-line therapy. Presents the IPSS reduction after various the second-line therapies following failure. This analysis included patients who required further intervention due to the initial PAE not achieving the desired outcomes. The graph shows a significant improvement in IPSS following ablative interventions (p < 0.0001; 95% CI: 12.75, 18.74). Error Bars: represent the SD, indicating the variability in IPSS reduction among patients undergoing each type of the second-line therapy.
IPSS reduction at least 12 months after the second-line therapy. Presents the IPSS reduction after various the second-line therapies following failure. This analysis included patients who required further intervention due to the initial PAE not achieving the desired outcomes. The graph shows a significant improvement in IPSS following ablative interventions (p < 0.0001; 95% CI: 12.75, 18.74). Error Bars: represent the SD, indicating the variability in IPSS reduction among patients undergoing each type of the second-line therapy.
Kaplan-Meier Survival Analysis: 5-Year Reoperation-Free Rates
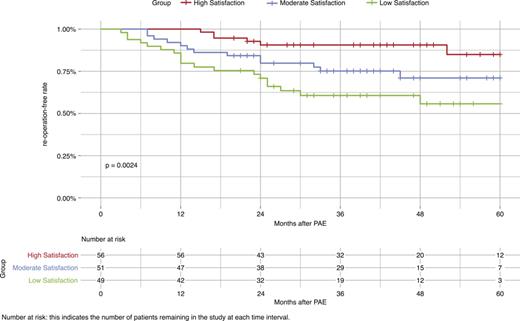
The log-rank test for Kaplan-Meier survival analysis (Fig. 4) demonstrated a statistical significance between patient satisfaction and the 5-year reoperation-free rate after PAE (p = 0.002). The high-satisfaction group showed an 85% reoperation-free rate at 60 months (95% CI: 72.9, 99.1), compared to 71% in the moderate-satisfaction group (95% CI: 58.2, 86.6), and 55.7% in the low-satisfaction group (95% CI: 41.6, 74.6).
Displays the Kaplan-Meier survival curves for 5-year reoperation-free rates among different patient satisfaction groups post-PAE. A significant correlation between patient satisfaction levels and reoperation-free survival was observed (p = 0.0024). The high satisfaction group exhibited an 85% reoperation-free rate at 60 months (95% CI: 72.93–99.07), in comparison to 71% for the moderately satisfied group (95% CI: 58.24–86.56), and 55.7% for the lowly satisfied group (95% CI: 41.6–74.6).
Displays the Kaplan-Meier survival curves for 5-year reoperation-free rates among different patient satisfaction groups post-PAE. A significant correlation between patient satisfaction levels and reoperation-free survival was observed (p = 0.0024). The high satisfaction group exhibited an 85% reoperation-free rate at 60 months (95% CI: 72.93–99.07), in comparison to 71% for the moderately satisfied group (95% CI: 58.24–86.56), and 55.7% for the lowly satisfied group (95% CI: 41.6–74.6).
Incidence of Incidental Prostate Cancer following Second-Line Therapy after PAE
Regarding the histological evaluation, 18.5% (5 of 27) of patients who underwent histological evaluation had incidental prostate cancer (iPCa), 4 had a Gleason score of 3 + 3 and 1 had a Gleason score of 4 + 3. The mean age (SD) of this group was 70.2 (5.7) years, and the median [IQR] PSA was 3.6 ng/mL [2.02–5.9].
Discussion
The management of BPH has evolved substantially, from the first detailed descriptions in the 16th century to the transformative TURP technique introduced in 1931 [15, 16], and now to less invasive options such as PAE. In this study, PAE resulted in an average IPSS reduction of 8 points, which, while effective, is less than the reduction achieved with TURP, as reported by Gilling et al. [17] (15.1 points) and Abt et al. [18] (9.21 points for PAE versus 12.09 for TURP). This indicates that while PAE offers symptom relief, it may not match the effectiveness of TURP for severe cases.
The patient satisfaction post-PAE varied, with younger patients and those with lower PVR volumes experiencing better outcomes. Older patients or those with higher PVR volumes showed reduced benefits and were more associated with an increased likelihood of PAE failure. This trend could be due to detrusor underactivity from prolonged obstruction, leading to weaker bladder contractions and higher residual volumes. A study by Bilhim et al. [19] supports these findings, noting a significant yet gradual reduction in prostate size after PAE, which may not sufficiently alleviate underlying bladder dysfunction. Meanwhile, patients with larger prostates reported greater satisfaction, possibly due to improved vascularization in larger prostates, thereby enhancing the effectiveness of embolization [20].
Despite many patients opting for PAE to preserve ejaculatory function, RE did not significantly correlate with patient satisfaction (p > 0.1) or PAE failure in our study. This suggests that, while maintaining ejaculatory function is a primary concern, other factors, such as symptom relief and quality-of-life improvements, may have a greater impact on overall satisfaction. Our study reports a 14% rate of RE after PAE, a rate that closely aligns with findings of Müllhaupt et al. [21].
One clinically significant observation was the failure rate associated with PAE. The 5-year reoperation rate after PAE was 28.2%. The only intervention reported in the literature to have a higher 5-year reoperation rate than PAE is transurethral microwave thermotherapy, which is documented to be 31.2% [22‒24]. The high failure rate, along with the need for careful patient selection and procedural refinement, could explain the observed differences in reoperation rates, as discussed by Altman et al. [25].
We found that ablative interventions, such as TURP or HOLEP, may be more effective as second-line therapies after PAE failure. This effectiveness is particularly important, as we observed a strong correlation between PAE failure and both elevated initial IPSS scores and higher PVR volumes (p < 0.01 and p = 0.03, respectively). These findings suggest that patients with more severe baseline symptoms, who are typically older with higher PVR volumes, may not be ideal candidates for PAE [26‒28].
In our study, we observed an 18% incidence rate of iPCa following secondary BPH surgeries, which is significantly higher than the approximately 10% rate typically reported after primary BPH surgeries [29]. This suggests that the initial symptoms in patients undergoing secondary surgeries may not have improved after PAE due to the presence of undiagnosed prostate cancer.
We acknowledge several limitations in our study. The retrospective collection of baseline data may introduce recall bias and inaccuracies. Additionally, the follow-up questionnaire was administered at a single time point, which may not fully capture long-term patient satisfaction and clinical outcomes. We did not include objective functional data such as uroflowmetry or PVR measurements, which limits our ability to correlate subjective outcomes with functional changes. While the IPSS is a validated tool for symptom assessment, the satisfaction scale we applied was not formally validated, which limits the reliability of these results. Conducting the study at a single center further limits the generalizability of the findings, as outcomes may vary across different healthcare environments and patient demographics. These factors should be considered when interpreting our findings.
To enhance our understanding of the factors that influence therapy failure and treatment outcomes after PAE, future research should use broader cohorts and advanced imaging techniques, such as MRI, to assess prostate morphology and functional changes. MRI can help identify early signs of therapy failure, improving patient selection and treatment planning. By studying vascular and tissue responses post-PAE, future studies can refine criteria to predict failure and enhance overall success. These approaches will increase understanding of why certain patients experience suboptimal outcomes after PAE.
Conclusion
PAE offers effective symptom relief for specific BPH patients, particularly younger individuals with larger prostates and lower PVR volumes, making them ideal candidates. However, nearly a third of patients may still require a second procedure, underscoring the importance of careful patient selection. Our study adds valuable evidence by showing that baseline IPSS and PVR volumes are important predictors of treatment failure post-PAE. Additionally, ablative interventions, such as HoLEP and TURP, may be more effective as second-line therapies than non-ablative options, but further research is needed to confirm these findings. The incidence rate of iPca after BPH surgery in our study was 18%, a factor that should not be underestimated.
Acknowledgments
This study was carried out in partial fulfilment of the requirements for the medical doctoral degree at the Medical Faculty, University Medical Center Hamburg-Eppendorf (UKE), University of Hamburg, Germany.
Statement of Ethics
The study received approval from the Ethics Committee of the Medical Association of North Rhine, Approval No. 2022330. All participants provided written informed consent after receiving information about the study’s objectives and potential risks and benefits.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by Helios health institute. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Author Contributions
K.A. organized the project, data collection, and wrote the first draft of the manuscript. This study was carried out in partial fulfilment of the requirements for the medical doctoral degree at the Medical Faculty, University Medical Center Hamburg-Eppendorf, University of Hamburg, Germany. P.E. and P.S. helped in project organization and data collection. W.A. and M.A. assisted and helped in organizing data collection for the project. M.F. and A.S. were senior organizing authors, helped in project conceptualization and assisted in manuscript drafting.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.