Abstract
Background: Historically, opening the bladder neck and urethra for evacuation has been solely by detrusor contraction with “urethral relaxation.” Summary: We present a change in thinking based on experimental research and clinical practice, human and animal: the urethra is opened externally immediately prior to detrusor contraction by posterior pelvic floor muscles contracting against competent uterosacral ligaments (USLs). A binary feedback control system (EITHER open OR closed) with neurological and peripheral musculo-ligamentous components is presented. Three oppositely acting muscle vectors contract against suspensory ligaments to close urethra distally and at bladder neck; relaxation of the forward closure vector allows the two posterior muscles to contract against USLs to open the posterior urethral wall prior to micturition. Whatever the anatomical cause of bladder emptying difficulties, neurological, muscle damage, ligament damage, urethral obstruction, inability of the pelvic muscles to externally open the urethra means the detrusor must contract against an unopened urethra which presents a high resistance to flow. This resistance is perceived by the patients as “bladder outlet obstruction.” Collagen damage during birthing, or breakdown after the menopause, makes ligament damage the most vulnerable part of the female micturition system. Treatment depends on reinforcing USLs as the contractile point for the posterior opening muscles: in premenopausal women by squatting-based exercises; in older women by mechanical support of USLs by pessary, or surgical USL repair. Key Message: A change in thinking: posterior pelvic muscles contract against a firm USL to open posterior urethral wall prior to micturition (video https://www.youtube.com/watch?v=nK0CQmaS-5E&t=7s).
Introduction
Hitherto, micturition was said to occur by bladder contraction and reciprocal urethral relaxation during voiding [1‒3] along with relaxation of the external (rhabdo) urethral sphincter and levator ani muscles [4]. No specific role was assigned to the pelvic muscles to directly open the urethra either prior to, or during micturition [1‒4]. For Zacharin, the function of the pelvic muscles was to support the pelvic organs from below [5].
Our aim was to demonstrate the importance of an external urethral/bladder neck opening mechanism for micturition, the role of exponentially determined urethral resistance to urine flow, pelvic ligament/muscle etiopathogenesis, and, based on these, corrective treatment, surgical, and nonsurgical.
External Closure of the Urethra by Pelvic Muscle Forces
In 1990, during development of the midurethral sling (MUS), X-ray, and ultrasound experiments demonstrated that 3 oppositely acting directional pelvic muscle forces (as shown by large arrows, Fig. 1), contracted against pubourethral ligaments (PUL) anteriorly and uterosacral ligaments (USL) posteriorly, to externally close the urethra distally and at bladder neck [6]. See X-ray frames 1 and 2 (as shown in Fig. 2, and video X-ray, https://www.youtube.com/watch?v=GkK5V6LvC3w). These discoveries explained how collagen created by the MUS [7] repaired PUL and restored the contractile closure forces of the urethra [8].
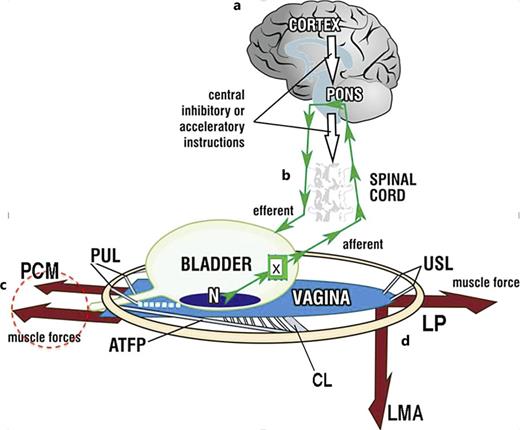
Binary model of bladder function. Schematic 3D sagittal view; system in normal closed mode. a Cortical binary control of OAB: afferent impulses “X” from stretch receptors “N” are reflexly suppressed cortically (white arrows). When required, the cortex activates the micturition reflex to evacuate bladder. b Peripheral binary control of continence is by a musculo‐ligamentous mechanism which responds to cortical efferents (small arrows) to stretch vagina in opposite directions to support “N” and decrease afferent impulses “X.” The three directional muscles (large arrows), forward, pubococcygeus muscle “PCM,” backward, levator plate “LP,” downward, conjoint longitudinal muscle of the anus “LMA” contract against the supporting ligaments, PUL (pubourethral) and USL (uterosacral), to stretch vagina tightly, much like the membrane of a drum. The stretched vagina supports the urine column, preventing activation of the stretch receptors “N,” decreasing afferent impulses to the cortex and premature activation of the micturition reflex (“urge to go,” urinary frequency). c Micturition: central control (white arrows) relaxes, as does PCM (broken circle) and rhabdosphincter (not shown); the posterior muscles LP and LMA open out the posterior wall of urethra (white broken lines) just prior to bladder evacuation. CX, cervix; CL, cardinal ligament; ATFP, arcus tendineus fascia pelvis. d Emptying dysfunction: weakness in the posterior muscles LP, LMA and/or the ligaments they contract against (USL), will affect the ability of LP/LMA to mechanically open the urethra (obstructed micturition), causing prolonged emptying times and slow flow, Fig. 3. However, other anatomical structures in Fig. 1, such as brain, nerve or direct muscle damage must always be considered to have a causative role.
Binary model of bladder function. Schematic 3D sagittal view; system in normal closed mode. a Cortical binary control of OAB: afferent impulses “X” from stretch receptors “N” are reflexly suppressed cortically (white arrows). When required, the cortex activates the micturition reflex to evacuate bladder. b Peripheral binary control of continence is by a musculo‐ligamentous mechanism which responds to cortical efferents (small arrows) to stretch vagina in opposite directions to support “N” and decrease afferent impulses “X.” The three directional muscles (large arrows), forward, pubococcygeus muscle “PCM,” backward, levator plate “LP,” downward, conjoint longitudinal muscle of the anus “LMA” contract against the supporting ligaments, PUL (pubourethral) and USL (uterosacral), to stretch vagina tightly, much like the membrane of a drum. The stretched vagina supports the urine column, preventing activation of the stretch receptors “N,” decreasing afferent impulses to the cortex and premature activation of the micturition reflex (“urge to go,” urinary frequency). c Micturition: central control (white arrows) relaxes, as does PCM (broken circle) and rhabdosphincter (not shown); the posterior muscles LP and LMA open out the posterior wall of urethra (white broken lines) just prior to bladder evacuation. CX, cervix; CL, cardinal ligament; ATFP, arcus tendineus fascia pelvis. d Emptying dysfunction: weakness in the posterior muscles LP, LMA and/or the ligaments they contract against (USL), will affect the ability of LP/LMA to mechanically open the urethra (obstructed micturition), causing prolonged emptying times and slow flow, Fig. 3. However, other anatomical structures in Fig. 1, such as brain, nerve or direct muscle damage must always be considered to have a causative role.
Mechanics of the urethral closure (continence) and opening (mcturition). X-ray 1: resting closed “S” denotes slow twitch muscle contraction of the three directional striated muscle forces, forwards PCM, backwards LP, downwards LMA (arrows) which contract against pubourethral ligament (PUL) anteriorly and uterosacral ligament (USL) posteriorly to close urethra distally and at bladder neck (6) CX, cervix; U, urethra; LP = levator plate muscle; PCM, pubococcygeus; LMA, conjoint longitudinal muscle of the anus; V, vagina; R, rectum; PB, perineal body. Radioactive dye has been inserted into vagina, rectum and levator plate. White broken lines = vertical and horizontal bony co-ordinates. X-ray 2: urethral closure during effort (coughing or straining). Compared to X-ray 1 (same patient), the forward muscle force (arrow) stretches the distal vagina (V) forwards against PUL to close the distal urethra, “urethral closure mechanism”; the backwards and downwards muscle forces (arrows), stretch and angulate the upper vagina and urethra around PUL to “kink” the proximal urethra, “bladder neck closure mechanism.” X-ray 3: micturition X-ray superimposed on resting xray patient sitting. Vascular clips have been applied to the midurethra “1.” Bladder neck “2” and bladder base “3.” Radio-opaque dye has been injected into the levator plate “LP,” which has been angulated downwards during micturition, as has the rectum “R,” which has 10 mL of barium paste. Broken lines indicate position of organs during micturition. Subscript “m” indicates the position of rectum “R” and levator plate “LP” during micturition. Note: backward extension of clips “2” and “3” indicating opening out of bladder neck and proximal urethra by LP/LMA contraction, and downward and forward movement of clip “1” indicating opening out of distal urethra by lateral/downward contracrtion of ischiocavernosus and bulbocavernosus muscles [9]. X-ray 4: the difference from straining (X-ray 2) is that the forward vector (“S” resting closed) relaxes. The backward/downward vectors stretch the vagina backwards and downwards against USL to open out the posterior urethral wall. The enlarged outlet exponentially decreases the resistance to evacuation by detrusor contraction inversely by the 4th power (Poiseuille’s Law). Comparing resting X-ray 1 with micturition X-rays 3 and 4: the distal urethra is pulled backwards from the vertical bony co-ordinate indicating PCM relaxation; angulation of the anterior portion of LP by downward LMA contraction pulls down rectum “R” and bladder base to “funnel” the bladder base and open the posterior wall of urethra.
Mechanics of the urethral closure (continence) and opening (mcturition). X-ray 1: resting closed “S” denotes slow twitch muscle contraction of the three directional striated muscle forces, forwards PCM, backwards LP, downwards LMA (arrows) which contract against pubourethral ligament (PUL) anteriorly and uterosacral ligament (USL) posteriorly to close urethra distally and at bladder neck (6) CX, cervix; U, urethra; LP = levator plate muscle; PCM, pubococcygeus; LMA, conjoint longitudinal muscle of the anus; V, vagina; R, rectum; PB, perineal body. Radioactive dye has been inserted into vagina, rectum and levator plate. White broken lines = vertical and horizontal bony co-ordinates. X-ray 2: urethral closure during effort (coughing or straining). Compared to X-ray 1 (same patient), the forward muscle force (arrow) stretches the distal vagina (V) forwards against PUL to close the distal urethra, “urethral closure mechanism”; the backwards and downwards muscle forces (arrows), stretch and angulate the upper vagina and urethra around PUL to “kink” the proximal urethra, “bladder neck closure mechanism.” X-ray 3: micturition X-ray superimposed on resting xray patient sitting. Vascular clips have been applied to the midurethra “1.” Bladder neck “2” and bladder base “3.” Radio-opaque dye has been injected into the levator plate “LP,” which has been angulated downwards during micturition, as has the rectum “R,” which has 10 mL of barium paste. Broken lines indicate position of organs during micturition. Subscript “m” indicates the position of rectum “R” and levator plate “LP” during micturition. Note: backward extension of clips “2” and “3” indicating opening out of bladder neck and proximal urethra by LP/LMA contraction, and downward and forward movement of clip “1” indicating opening out of distal urethra by lateral/downward contracrtion of ischiocavernosus and bulbocavernosus muscles [9]. X-ray 4: the difference from straining (X-ray 2) is that the forward vector (“S” resting closed) relaxes. The backward/downward vectors stretch the vagina backwards and downwards against USL to open out the posterior urethral wall. The enlarged outlet exponentially decreases the resistance to evacuation by detrusor contraction inversely by the 4th power (Poiseuille’s Law). Comparing resting X-ray 1 with micturition X-rays 3 and 4: the distal urethra is pulled backwards from the vertical bony co-ordinate indicating PCM relaxation; angulation of the anterior portion of LP by downward LMA contraction pulls down rectum “R” and bladder base to “funnel” the bladder base and open the posterior wall of urethra.
External Opening of the Urethra by Pelvic Muscle Forces-Role of USL
Also, described in 1990 [6], was a new concept for micturition, which was enlargement of the outflow channel by external opening of the urethra during micturition by the two posteriorly acting pelvic muscles, frames 3 and 4 (as shown in Fig. 2; video https://www.youtube.com/watch?v=nK0CQmaS-5E&t=7s).
In 1993, the term “Posterior Fornix Syndrome” (PFS) was introduced to describe a predictable cluster of symptoms, including urgency, frequency, nocturia, chronic pelvic pain, abnormal bladder emptying, and post-void residual urine. These symptoms were attributed to USL laxity and were shown to be cured or improved through USL repair [10].
The PFS discovery led to extensive video X-ray myogram studies, and also, cadaveric studies, to identify the four major pelvic muscles, pubococcygeus (PCM), levator plate (LP), LMA (conjoint longitudinal muscle of the anus), and puborectalis (PRM) [11, 12], and their roles in functional bladder anatomy (as shown in Fig. 2).
Dye injected into the bladder, vagina, rectum, and levator plate demonstrated that the posterior urethral wall was actively opened during micturition by the action of backward (LP) and downward (LMA) muscle forces contracting against USL (as shown by X-ray frames 3 and 4, micturition, Fig. 2) [11‒13].
In a 1997 study, the key role of PUL and USL for bladder function was tested directly by pre and postoperative urodynamics, and cough and 24-h pad tests [14]. High cure rates were recorded for stress urinary incontinence (SUI) and PFS bladder symptoms (urge, frequency, nocturia) with improvement of emptying symptoms and post-void residual urine [14].
External Opening of the Urethra by Pelvic Muscle Forces-Role of Perineal Muscles
During extensive videomyogram studies of peripheral musculo/ligamentous bladder closure and evacuation, some with vascular clips applied to the midurethra, bladder neck, and bladder base [11‒13], it was observed that clip “1” (as shown in by frame 3, Fig. 2) was pulled down distally during micturition. The downward movements were attributed to lateral and downward contraction of m.ischiococcygeus (IC) and m.bulbocavernosus (BC). In an elegant cystometrogram and EMG study in female rabbits, Corona-Quintanilla et al. [9] confirmed the human study findings [11‒13]: no EMG was recorded during micturition in m.pubococcygeus (indicating PCM relaxation); IC and BC contracted throughout micturition (indicating active opening of distal urethra). Furthermore, IC activation coincided with observed lateral movement of the clitoral sheath which would have expanded the distal urethra posteriorly (as shown in frame 3, Fig. 2).
Bladder Control Is Binary
In 1999, anomalous urodynamic findings during an experiment on low compliance bladder led to discovery of a binary cortico-peripheral feedback system of bladder control [15] (as shown by Fig. 1). The binary system has only two modes, EITHER open OR closed, with closed being dominant. Three oppositely‐acting striated pelvic muscles contract reflexly against competent PUL and USL ligaments, tensioning the vagina like a trampoline to support the urothelial stretch receptors “N” from below (as shown by Fig. 1), [15].
Cortical Control of Urethral Closure
With reference to Figure 1, as the hydrostatic pressure of the urine presses on “N,” afferent impulses to the brain increase and are interpreted as “urge to go.” If inconvenient to empty, the brain blocks these impulses (white arrows). At the same time, the vaginal membrane supporting “N” is reflexly stretched by the increase in contractile force by oppositely acting PCM, LP, and LMA, to decrease the afferent impulses “X” to the pons [15].
Cortical Control of Urethral Opening Prior to Detrusor Contraction
When the cortex decides it is appropriate to evacuate the bladder, the dominant closure reflex is totally suppressed, and the forward vector PCM (pubococcygeus) relaxes (as shown in Fig. 1, 2) [11‒13, 16]; also relaxing is the rhabdosphincter (periurethral striated muscles) [5, 13]. With reference to Figure 2, PCM relaxation removes the backward pressure of the vagina on the posterior urethral wall; this allows LP to stretch the proximal vagina backwards against PUL to tension it into a semirigid state, much like a trapdoor; the LMA pulls down the proximal vagina, bladder base and posterior urethral wall to open out the outflow tract (as shown by frames 3 and 4, Fig. 2, and the video at https://www.youtube.com/watch?v=nK0CQmaS-5E&t=7s).
LP/LMA expansion of urethral diameter (as shown in frames 3 and 4, Fig. 2), exponentially decreases urethral resistance to detrusor emptying by a power of 4 (Poiseuille's Law) (as shown by Fig. 3). Because electrical transmission for the detrusor is from smooth muscle to smooth muscle [17], the detrusor smooth muscles contract and shorten as a singular mass until the bladder is empty. The slow shrinking of the detrusor as it empties is visually evident in the dynamic X-ray (2nd part) of the micturition (video https://www.youtube.com/watch?v=nK0CQmaS-5E&t=7s).
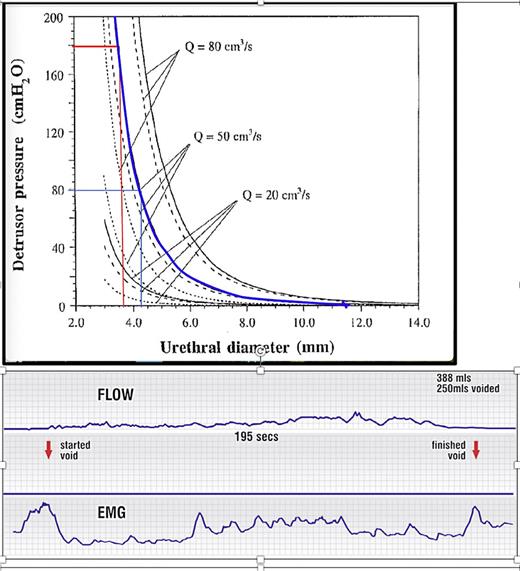
Narrowed urethra causes “obstructed micturition”: raised resistance, emptying pressure, and low flow. Upper figure: detrusor pressure at 3 different flow rates as a function of the urethral diameter for a tube length of 4 cm: frictional component: dotted line; dynamic component: dashed line; total: solid line. Blue curve: resistance to urine flow of 50 mL/s in the urethra, is exponentially determined, by the diameter of the urethra. Note: even a 0.5 mm change from 3.7 mm to 4.2 mm in urethral diameter reduces the detrusor pressure required to urinate at 50 mL/s from 180 cm H2O to 80 cm H2O. Opening to 6.0 mm (2.3 mm change) reduces detrusor pressure required to 20 cm H2O. Horizontal axis, urethral diameter; vertical axis = detrusor pressure required to drive the urine through the urethra. Lower figure: flow chart in a woman with “obstructed micturition” and an EMG electrode in the posterior fornix of vagina, recording contraction of the posterior opening muscles (LP and LMA). The EMG is activated before urine flow commences. Note continuous activation of the pelvic floor muscles.
Narrowed urethra causes “obstructed micturition”: raised resistance, emptying pressure, and low flow. Upper figure: detrusor pressure at 3 different flow rates as a function of the urethral diameter for a tube length of 4 cm: frictional component: dotted line; dynamic component: dashed line; total: solid line. Blue curve: resistance to urine flow of 50 mL/s in the urethra, is exponentially determined, by the diameter of the urethra. Note: even a 0.5 mm change from 3.7 mm to 4.2 mm in urethral diameter reduces the detrusor pressure required to urinate at 50 mL/s from 180 cm H2O to 80 cm H2O. Opening to 6.0 mm (2.3 mm change) reduces detrusor pressure required to 20 cm H2O. Horizontal axis, urethral diameter; vertical axis = detrusor pressure required to drive the urine through the urethra. Lower figure: flow chart in a woman with “obstructed micturition” and an EMG electrode in the posterior fornix of vagina, recording contraction of the posterior opening muscles (LP and LMA). The EMG is activated before urine flow commences. Note continuous activation of the pelvic floor muscles.
Evacuation Pathogenesis – Collagen Is the Achilles Heel
Theoretically, anatomical damage or dysfunction in any component of the binary model (as shown by Fig. 1), [15], can cause emptying difficulties: extrinsic pressure on stretch receptors “N” e.g., by enlarged prostate in the male, ligament weakness, pelvic muscle damage, neurological injury (afferent/efferent nerve, spinal cord, brain), or overtight MUS tape blocking the urethral lumen. The degree of imbalance depends on site and extent of damage. Because collagen may be damaged at birth or by age, weak ligaments are the Achilles heel in the system, and so can be considered as a major cause of bladder dysfunction [15, 16].
The Role of Urethral Resistance in Obstructed Micturition
Figure 3 presents the graphic relationship between urethral diameter and urine flow of 20, 50, and 80 mL/s. Contraction of the detrusor against a urethra which cannot be fully opened by the external pelvic muscle mechanism, is perceived by the patient as “obstructive micturition.” Because the urethral resistance to flow is exponentially determined, being inversely proportional to the 4th power of the radius (Poiseuille’s Law (18–22) (as shown by Fig. 3), even a minor vaginal prolapse, i.e., loose ligaments, can weaken the pelvic muscles which contract against USLs to cause significant emptying difficulties. Minor inability to externally open the urethra from 3.7 mm to 4.2 mm (only ½ mm!) (as shown by Fig. 3), more than halves the detrusor expulsion pressure required for 50 mL/s from 180 cm H2O to 80 cm. If the urethra can be opened to 6 mm, the detrusor expulsion pressure falls to 20 cm H2O.
The flow chart (as shown in Fig. 3) is from a woman with “obstructed micturition”: the detrusor contracts against a urethra which cannot be fully opened by the external pelvic muscle mechanism. The EMG electrode in the posterior fornix of the vagina, records the contraction of the posterior opening muscles (LP and LMA). The EMG graph (as shown by Fig. 3) is activated before urine flow commences, indicating that pelvic muscle contraction precedes urine flow. The slow prolonged urine flow and constant activation of the pelvic floor muscles can be attributed to the fast-twitch muscles repeatedly trying to open the outflow channel.
Objective Flow Measurements Are Rarely Exactly Repeatable
The exponential nature of the graph, Figure 3, explains major differences found with peak flow measurements when they are repeated on another occasion: any minor changes in input from the multiple anatomical contributors from the binary feedback model (as shown by Fig. 1), can be exponentially magnified to deliver a different flow reading. In a 1998 study [20], women with initial micturition pressures of 8, 10, 16, 20, 20 cm H2O had pressures of 16, 50, 30, 26, and 12 cm H2O when repeated at a later date. In an exponentially determined relationship (as shown in Fig. 3), only a very minor difference in urethral diameter is required to show a different detrusor pressure/flow relationship.
Weakening of LP/LMA Opening Forces by Uterine Prolapse
Fundamental to why loose USLs can cause weakened urethral opening forces, is that a sarcomere contracts optimally only over a short length “L”* [21] (as shown by Fig. 4.). *With reference to Figure 4, even at length “L,” there is 80% contractile force available, not 100%. At double “L,” there is only 30% force available to externally open the posterior wall of the urethra prior to micturition.
Lax uterosacral ligaments diminish the contractile strength of pelvic muscles which contract against them. a Upper figure: the weak USLs elongated to e, result in elongation of LP and LMA (which contract against USLs) also to “E.” Loose USLs weaken muscle forces of LP and LMA needed to open the urethra. b Lower figure: interpretation based on known sarcomere physiology according to Gordon [21]. Striated muscles (LP/LMA) contract optimally over a short length only of the sarcomere (L, red rectangle). Elongation of muscles to e (black rectangle) cause rapid loss of contractile muscle force needed to open the urethra. If loose USLs weaken the LP and LMA muscle forces needed to open the urethra, the detrusor must contract against an unopened urethra, with higher pressure, prolonged flow, high residual.
Lax uterosacral ligaments diminish the contractile strength of pelvic muscles which contract against them. a Upper figure: the weak USLs elongated to e, result in elongation of LP and LMA (which contract against USLs) also to “E.” Loose USLs weaken muscle forces of LP and LMA needed to open the urethra. b Lower figure: interpretation based on known sarcomere physiology according to Gordon [21]. Striated muscles (LP/LMA) contract optimally over a short length only of the sarcomere (L, red rectangle). Elongation of muscles to e (black rectangle) cause rapid loss of contractile muscle force needed to open the urethra. If loose USLs weaken the LP and LMA muscle forces needed to open the urethra, the detrusor must contract against an unopened urethra, with higher pressure, prolonged flow, high residual.
Figure 4 pictorially demonstrates how USLs elongated by length “E” cause uterine prolapse and, at the same time, weaken the opening forces of LP/LMA. Because LP/LMA effectively contract against USLs, they also lengthen by “E” when the uterus prolapses. At a length “L” plus “E,” contractile strength weakens considerably, indicated by wavy arrows [21]. The urethra cannot be opened adequately, and the detrusor has to contract against a relatively unopened urethra, requiring increased detrusor pressure and longer emptying times (as shown by Fig. 3). The weakening of detrusor contractile force, analysed in Figures 3 and 4, is described in some journals as “UAB” (underactive bladder). Its cause, like “BOO” (bladder outlet obstruction), is said to be unknown. Our anatomical analysis suggests “BOO,” “UAB,” “obstructed micturition” are all the same pathogenic entity.
Anatomical Pathway for BOO Cure by USL Repair for Uterine/Apical Prolapse
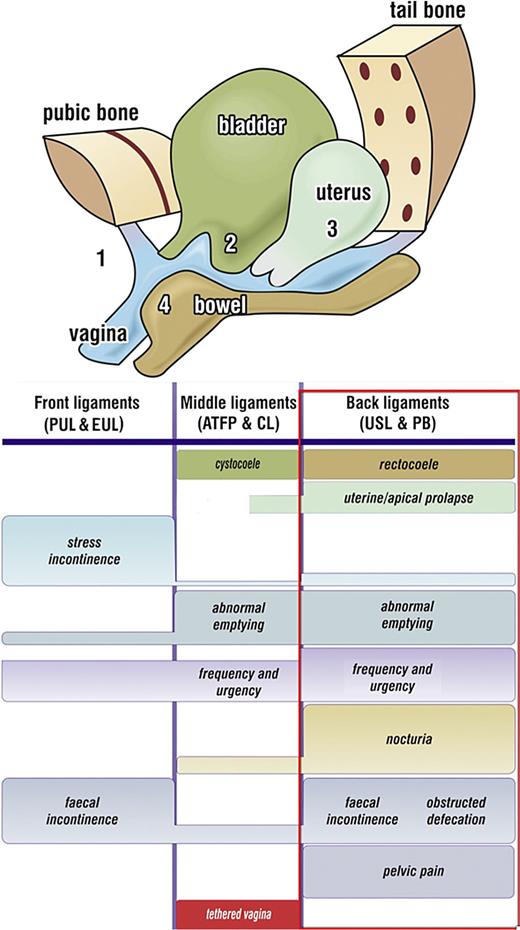
Figure 4 shows how USL laxity weakens LP/LMA, which open the posterior urethral wall. The studies quoted [22‒32] show cure of BOO treated women with varying stages of uterine/apical prolapse (some stage 1) by USL repair. The studies reported high, but different cure or improvement rates for prolapse and each of the “Posterior Fornix Syndrome” (PFS) symptoms (as shown by rectangle, Fig. 5) (chronic pelvic pain, urge, frequency and nocturia, emptying), indicating a different anatomical pathway for each symptom.
Diagnostic algorithm. The rectangle represents the co-occurring symptoms of the “Posterior Fornix syndrome.” CPP and nocturia are uniquely caused by uterosacral ligament (USL) laxity and are cured or improved by USL repair.
Diagnostic algorithm. The rectangle represents the co-occurring symptoms of the “Posterior Fornix syndrome.” CPP and nocturia are uniquely caused by uterosacral ligament (USL) laxity and are cured or improved by USL repair.
As a detailed example of the above statement, we quote a pre and postoperatively monitored urodynamic study of 24 women treated by a 7 mm mesh USL minisling [25]. Mean pre-op results are compared with post-op data (in brackets): PVR (post-void residual) 272 mL (34 mL); abnormal emptying symptoms n = 24 (18/24 cured or 80% improved); natural bladder volume 598 mL (301 mL); emptying time 50 s (20 s): peak flow 42 mL/s (37 mL/s); chronic pelvic pain n = 18 (14/18 >80% improved); 4 women with high maximal urethral closure pressure reduced from mean 93 cm H2O to 75 cm; urinary frequency (14/14 improved); nocturia 110 episodes reduced to 33 episodes.”
Discussion
The external musculoligamentous mechanism of bladder control based on collagen-weakened ligaments was initially presented as a hypothesis in 1990, with only limited evidence in [1]. The hypothesis has been subsequently challenged extensively and deductively, by X-ray and ultrasonic imaging, urodynamic studies [6‒8, 10‒14], animal studies [9, 17, 21] mathematical and flow mechanics studies [18‒20, 33, 34] and surgically, by observing changes after repair of the uterosacral and PULs [22‒32, 35‒41].
Simplistically, with reference to Figure 2, we can reduce the binary function of the bladder to one single element, urethral resistance. Both binary functions, urethral closure and evacuation, depend on urethral resistance (as shown by Fig. 3): small urethral diameter during closure = high resistance to flow (continence); enlarging the urethral outlet during micturition = low resistance to flow and ease of evacuation. Anything which obstructs urethra, or weakens the contractile strength of LP/LMA may cause emptying difficulties (“obstructed micturition”). Existing concepts of normal and obstructed micturition causation and treatment are briefly analysed in this context.
Analysis of Urethral Kinking/Compression Hypotheses as Cause of BOO
Our data does not support urethral kinking/compression hypotheses as cause of BOO. With very little evidence, such “pressure” hypotheses posit that the weight of a cytocele on the urethra would obstruct the urethra to cause obstructed micturition. This hypothesis was invalidated by Bush et al. [19] who calculated that a water column 160 metres high would be required for abdominal pressure to funnel the bladder neck for micturition to take place. It was more directly invalidated by Himmler et al. [23] in a prospective symptom observation study of women who had mesh supported posterior ligament sacrospinal fixation for various prolapse conditions. They demonstrated that women treated primarily for rectoceles and cystoceles had similar preoperative prevalences of obstructive micturition symptoms (BOO) even when there was minimal apical descent [23]. They also showed that in all such subgroups, high BOO symptom cure rates up to 24 months postoperatively could be observed following anchoring of the mesh to the sacrospinous ligament.
In a retrospective study of 190 women with 2nd and 3rd degree prolapse, 23 were selected with isolated posterior compartment defect [35]; 13 of these (56%) had BOO with isolated large rectoceles, but no cystoceles. Eight of these women had rectocele repair, and 7 reported cure of their BOO [35]. These here presented results [23, 35] fit with our concept of a firm posterior anchoring point for the external opening mechanism, but not by prolapse-associated urethral kinking or urethral compression.
Urethral Relaxation during Micturition – A Critical Analysis
A commonly cited hypothesis for micturiton is that “the parasympathetic fibres inhibit the internal urethral sphincter, which causes relaxation allowing for bladder emptying” [36].
The longitudinal smooth muscles of the detrusor form a continuum with the urethra, so the urethra and bladder function as a unit in continence as well as during voiding [37]. It is not anatomically possible for the urethral smooth muscle to “relax” at the same time as the detrusor contracts when they both share the same smooth muscle continuum.
The fall in urethral pressure observed prior to micturition has been attributed to relaxation of the striated rhabdosphincter [5], and also, to relaxation of PCM [38]. However, the statement “total relaxation of the levator” [5] is not supported by EMG findings, Figure 3 [42, 43]. A cylindrical EMG probe placed in the posterior vagina fornix demonstrates posterior pelvic muscle contraction prior to and during micturition (as shown in graph, Fig. 3). Furthermore, comparing frames 1 and 4 Figure 2 (resting closed and micturition), “levator muscle relaxation” [5] seems confined only to PCM, as LP/LMA evidently pull the vagina and bladder base backwards and downwards to externally open the outflow tract [11‒13, 18‒20, 33, 34].
Relaxation Muscle Therapy for Functional BOO
The concept of “pelvic floor relaxation” during micturition [36] was directly tested by Bo who found it did not improve patients who had difficulty in bladder evacuation [44]. The mechanics of external opening (as shown in Fig. 2, 3) predict that pelvic relaxation exercises would have a poor outcome. This was found to be so [44]. However, using a squatting-based regime which aims to strengthen the three reflex muscle forces and the PUL and USL ligaments against which they contract, Skilling found 50% improvement in multiple pelvic symptoms (including BOO), with major reductions in post-void residual urine, Table 1 [45].
Fate of individual symptoms (n = 78)
| Fate of individual symptoms . | Condition, n . | >50% improvement, n [%] or mean . |
|---|---|---|
| Stress incontinence | 69 | 57 [82] |
| Urge incontinence | 44 | 33 [68] |
| Frequency only | 12 | 10 [83] |
| Nocturia | 32 | 29 [90] |
| Pelvic pain | 17 | 13 [76] |
| Residual urine mean 202 mL | 23 | Mean 71 mL |
| Fate of individual symptoms . | Condition, n . | >50% improvement, n [%] or mean . |
|---|---|---|
| Stress incontinence | 69 | 57 [82] |
| Urge incontinence | 44 | 33 [68] |
| Frequency only | 12 | 10 [83] |
| Nocturia | 32 | 29 [90] |
| Pelvic pain | 17 | 13 [76] |
| Residual urine mean 202 mL | 23 | Mean 71 mL |
Urethral Incision for Obstructed Micturition
Botulinum Toxin Injected at Bladder Neck
Though promising reports of this method have been reported, a randomized, double-blind, placebo-controlled study with an improvement rate of 43%, showed little clinical difference between placebo and OnabotulinumtoxinA. The placebo, in some aspects registered a greater therapeutic effect [40].
Surgical Repair of Cardinal/Uterosacral Ligaments when BOO Is Part of Posterior Fornix Syndrome
The data reported for cure of Posterior Fornix Syndrome (PFS) symptoms [22‒32] (as shown in rectangle, Fig. 5), was mainly using a posterior USL sling. However, very acceptable cure rates for uterine/apical prolapse and three PFS symptoms (urge, frequency, nocturia) at 18 months have been reported with native ligament USL and CL (cardinal ligament) repair**, but only in premenopausal women [41, 46], as the cure rates at 18 months in post-menopausal women were catastrophically low. The authors recommended use of a collagen-creating technique (e.g., slings) in post-menopausal women.
** Essentially a simplified Manchester operation, with CL and USL repair, with conservation of the uterus and vaginal tissue. Our 30-year experience with ligament repairs confirms that a simplified Manchester operation would help improve BOO if it is part of the Posterior Fornix Syndrome (as shown in rectangle, Fig. 5). In post-menopausal women, we recommend use of collagen-creating No.2 polyester sutures applied to CL and USL [47] (see video at https://www.youtube.com/watch?v=pEa61sWHkaQ).
Conclusion
We have demonstrated a change in thinking for micturition in the female: that posterior pelvic muscles contract against firm USLs to open the posterior urethral wall prior to micturition; that lax USLs weaken external opening of the urethra to cause bladder outlet obstruction (BOO); that repair of USLs can achieve substantial cure rates for BOO. It is our view that USL surgery is not the full pathogenesis story. Clearly severely damaged LP/LMA muscles must diminish the contractile opening forces. Nor is it the full therapeutic story. The stated etiopathogenesis necessarily extends to any non-surgical method which strengthens or mechanically supports USLs. Squatting-based exercises have recorded 50% improvement in emptying symptoms and reduction of post-void residual urine, from 202 mL to 71 mL. It is well known that vaginal pessaries, large tampons and in Germany, 3 × 6 cm cylindrical probes can improve Posterior Fornix Syndrome symptoms which include BOO. All are fertile fields for future studies.
Conflict of Interest Statement
A related entity to Peter Emanuel Petros (Control Plus) has applied for a patent on a pelvic floor device, but neither the entity nor Peter Emanuel Petros has received any remuneration. Bernhard Liedl, Maren Juliane Wenk, and Paolo Palma have no conflicts to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Conceptualization: P.E.P., B.L., writing original draft: P.E.P., B.L. Writing, reviewing, editing: P.E.P., B.L., M.J.W., and P.P. Final review and approval: P.E.P., B.L., M.J.W., P.P. Resources, figures, and table P.E.P.


![Mechanics of the urethral closure (continence) and opening (mcturition). X-ray 1: resting closed “S” denotes slow twitch muscle contraction of the three directional striated muscle forces, forwards PCM, backwards LP, downwards LMA (arrows) which contract against pubourethral ligament (PUL) anteriorly and uterosacral ligament (USL) posteriorly to close urethra distally and at bladder neck (6) CX, cervix; U, urethra; LP = levator plate muscle; PCM, pubococcygeus; LMA, conjoint longitudinal muscle of the anus; V, vagina; R, rectum; PB, perineal body. Radioactive dye has been inserted into vagina, rectum and levator plate. White broken lines = vertical and horizontal bony co-ordinates. X-ray 2: urethral closure during effort (coughing or straining). Compared to X-ray 1 (same patient), the forward muscle force (arrow) stretches the distal vagina (V) forwards against PUL to close the distal urethra, “urethral closure mechanism”; the backwards and downwards muscle forces (arrows), stretch and angulate the upper vagina and urethra around PUL to “kink” the proximal urethra, “bladder neck closure mechanism.” X-ray 3: micturition X-ray superimposed on resting xray patient sitting. Vascular clips have been applied to the midurethra “1.” Bladder neck “2” and bladder base “3.” Radio-opaque dye has been injected into the levator plate “LP,” which has been angulated downwards during micturition, as has the rectum “R,” which has 10 mL of barium paste. Broken lines indicate position of organs during micturition. Subscript “m” indicates the position of rectum “R” and levator plate “LP” during micturition. Note: backward extension of clips “2” and “3” indicating opening out of bladder neck and proximal urethra by LP/LMA contraction, and downward and forward movement of clip “1” indicating opening out of distal urethra by lateral/downward contracrtion of ischiocavernosus and bulbocavernosus muscles [9]. X-ray 4: the difference from straining (X-ray 2) is that the forward vector (“S” resting closed) relaxes. The backward/downward vectors stretch the vagina backwards and downwards against USL to open out the posterior urethral wall. The enlarged outlet exponentially decreases the resistance to evacuation by detrusor contraction inversely by the 4th power (Poiseuille’s Law). Comparing resting X-ray 1 with micturition X-rays 3 and 4: the distal urethra is pulled backwards from the vertical bony co-ordinate indicating PCM relaxation; angulation of the anterior portion of LP by downward LMA contraction pulls down rectum “R” and bladder base to “funnel” the bladder base and open the posterior wall of urethra.](https://karger.silverchair-cdn.com/karger/content_public/journal/uin/pap/10.1159_000547145/1/m_000547145_f02.jpeg?Expires=1772283916&Signature=DyaP6-vhcutKj5iHtZYSiccakvPNhpNkKLK2yaOmlSgnHRdQGjPr4~scrZKWk5E4DYyVkWYfhoclA8NSEEal8lw6kOjPPoKrjdauHqHScqVSG9BJErYXeAwK7pw2SV4DcEx8DMfBKbq2ieBwOX4UasPoWfCB0O9l7UM3d5CrytuduNjjRsBBTnybL5PxPGp7A8oYKByMy9OlGN4ET7wdikZjxKui-7tm312IHHL1NUxXr8KQ6YuLPO96wkal5IRAkje4Se0dEduG4oDf5Hfo2g4~Hd6T~1aWBfEdH8dXCbahYC2gSNugjwJECGXwWo7h6aEeWjyGJEyZG4UAXXW0nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Lax uterosacral ligaments diminish the contractile strength of pelvic muscles which contract against them. a Upper figure: the weak USLs elongated to e, result in elongation of LP and LMA (which contract against USLs) also to “E.” Loose USLs weaken muscle forces of LP and LMA needed to open the urethra. b Lower figure: interpretation based on known sarcomere physiology according to Gordon [21]. Striated muscles (LP/LMA) contract optimally over a short length only of the sarcomere (L, red rectangle). Elongation of muscles to e (black rectangle) cause rapid loss of contractile muscle force needed to open the urethra. If loose USLs weaken the LP and LMA muscle forces needed to open the urethra, the detrusor must contract against an unopened urethra, with higher pressure, prolonged flow, high residual.](https://karger.silverchair-cdn.com/karger/content_public/journal/uin/pap/10.1159_000547145/1/m_000547145_f04.jpeg?Expires=1772283916&Signature=IqjxwmrSolFIJa8OxD1H0LTcKfUMULN9FiLmrgdSB4K~EdAg77ctuU-IQT7CrrKayQy6DlpRpmcF9IxF4kbKlu8vtMY6eaGha3VCjKeqORdeW5K5tR-UyTujDtoJL2EB87ITkvtU1qCXIUO11T8O72bK7EZAqgAPu6zcAbh6Fh1p89-ZWzS54nIu7I2JQsbuJuwLPezB1JipMlknjNH1H0tlWPj7PZSe1HXAnnKQvJKe5vPppidzKLiOUaA5VS6QfXKi-H8bV0gVNlFx~5s3exSPIr~MqaN8MUgkiO19iKHgLhSqrNv~M0C6scuhBot~xmbovPv~cOzKVuL1UatrOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)