Abstract
Introduction: Erectile dysfunction is a debilitating condition defined as the inability to achieve or maintain penile erection sufficient for satisfactory sexual activity. Obstructive sleep apnoea is a common sleep disorder resulting in episodes of intermittent hypoxia experienced by the patient. It has been hypothesized that a link between both conditions exists. This systematic review aims to evaluate the current literature and synthesize the evidence linking erectile dysfunction and obstructive sleep apnoea, the possible pathogenesis involved, and potential treatment options available. Methods: This is a mixed-method systematic review and narrative synthesis reporting qualitative and quantitative data. The databases MEDLINE, PubMed, and Embase were searched between January 2000 and January 2022. Results: Eighteen studies were included, comprising 5 randomized controlled trials, 11 prospective studies, and 2 cross-sectional studies. The literature has reported up to 82% prevalence of ED in patients with OSA. Studies investigating the role of CPAP in treating ED in this cohort of patients have shown results varying from no significant effect to complete resolution of ED. Conclusion: There does appear to be an association between the presence of OSA and ED. Further randomized controlled trials to evaluate the efficacy of CPAP on ED in patients with OSA need to be conducted. Until more robust evidence is available, CPAP is a safe treatment likely to improve symptoms in individuals with OSA. However, the degree to which it may affect ED symptoms is not yet clear, but what appears clear is that it does not negatively impact on erectile function and is most likely to offer some degree of improvement.
Introduction
Erectile dysfunction (ED) is a debilitating condition defined as the inability to achieve or maintain penile erection sufficient for satisfactory sexual activity [1]. It is one of the most common sexual conditions amongst men, with an estimated 324,600 cases in the UK [2]. This number is thought to be increasing in recent years. In the past, ED was thought to be a psychological disorder; however more recently, we know it to be of multifactorial aetiology, of which vasculogenic, neurogenic, and endocrine aetiologies are most prevalent [3]. Vascular disease resulting in endothelial dysfunction and neurogenic ED involves a failure in the neuronal communication with the corpus cavernosa. This reduces the amount of nitrous oxide available to the vascular smooth muscles, impeding vasodilation and thus impairing erection. The endocrine aetiology focuses on androgens and the importance of testosterone. Testosterone partly contributes via its ability to provide the sex drive in males. In addition to this, testosterone has been shown to modulate the release of nitric oxide in endothelial cells and phosphodiesterase 5 in smooth muscle [4].
Obstructive sleep apnoea (OSA) is a common sleep disorder resulting in episodes of intermittent hypoxia experienced by the patient [5]. Apnoea hypoxia index (AHI) is the number of apnoea episodes per hour and is used to determine the severity of OSA with normal <5/h, mild 5–15/h, moderate 15–30/h, severe >30/h. It has been documented in the literature that OSA has been implicated in the development of microvascular complications, which if left uncontrolled can have serious consequences involving the nervous system and vasculature. Hypoxia itself has been shown to be responsible for decreasing the levels of prostaglandin E1, which ordinarily inhibit pro-fibrotic cytokines. This results in increased collagen deposition, decreasing penile elasticity and erectile quality [6]. In addition, with increased collagen content, the corpus cavernosa loses its ability to compress the subtunical veins leading to veno-occlusive dysfunction and incomplete tumescence.
This systematic review aims to evaluate the current literature and summarize the evidence linking ED and OSA, the possible pathogenesis involved, and potential treatment options available. Although reviews have looked at this topic previously, they have now become outdated and we aim to provide an updated comprehensive overview of the available evidence.
Methods
Design
This mixed method systematic review using narrative synthesis was conducted in agreement with the guidelines set out by the Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) statement (http://www.prisma-statement.org). The PRISMA checklist is included as supplementary material (for all online suppl. material, see https://doi.org/10.1159/000546690).
Search Strategy
Preliminary searches of the existing English language literature were conducted in January 2022 using the NICE Healthcare Databases Advanced Search (https://www.evidence.nhs.uk) to gather search terms and keywords. Extensive searches were conducted using databases including MEDLINE (1946–present), PubMed (1946–present), and Embase (1974–present). Additional relevant studies were incorporated from the bibliography of review articles, in addition to studies obtained via the databases.
Study Eligibility Criteria
Initial screening of titles and abstracts was conducted by Z.A.S. and these were further reviewed by Z.A.S., D.P.M., A.S., I.P., and V.M. to assess for inclusion based on the following inclusion criteria:
Population: studies must include patients suffering with ED and OSA;
Intervention: studies must include data on the impact of OSA treatment with CPAP on ED;
Comparator: studies which report on the impact of standard practice OSA treatment for patients with ED and OSA;
Outcome: impact on severity of ED and OSA based on IIEF and AHI, respectively;
Publication: studies must be peer reviewed and published between January 2000 and January 2022. Studies published prior to January 2000 along with case reports, systematic reviews, book chapters, abstracts, opinion letters, and non-peer reviewed works were excluded from this systematic review. Randomized controlled trials, prospective and cross-sectional studies were all included.
Study Selection and Data Collection
Abstracts were reviewed and irrelevant studies were excluded. Full-text articles were studied by all authors for eligibility, identifying those studies that reported on prevalence of ED in patients with OSA and treatment options for patients in this cohort. Conflicts of opinion between all authors were discussed until there was agreement on eligibility for all included studies.
Quality Appraisal
Quality appraisal was conducted independently by ZAS and VM. Quality was assessed using the mixed methods appraisal tool (MMAT) using set criteria for each study design. Any conflicts of opinion were discussed amongst all authors until there was agreement. No study was discarded based on quality.
Data Extraction
Full-text articles were reviewed to extract relevant information to this review. Extracted data were tabulated for summary and analysis including author, title of study, year of publication, type of study, journal of publication, and main findings regarding treatment of OSA benefitting ED (Table 1).
Overview of studies included in this systematic review
| Author . | Title . | Journal . | Year of publication . | Type of study . | Findings . |
|---|---|---|---|---|---|
| Schneider et al. [7] | Influence of testosterone on breathing during sleep | Journal of Applied Physiology | 1987 | Prospective study | Both apnoeas and hypopnoeas increased significantly when hypogonadal men were treated with testosterone replacement |
| Granata et al. [8] | Relationship between sleep-related erections and testosterone levels in men | Journal of Andrology | 1997 | Prospective study | Serum testosterone levels for nocturnal erections were 200 ng/dL |
| Fanfulla et al. [9] | Erectile Dysfunction in Men with Obstructive Sleep Apnea: An Early Sign of Nerve Involvement | Sleep | 2000 | Prospective study | 68% of men with OSA had an altered BCR. These patients had higher AHI, higher percentage of sleep SaO2 <90, and lower daytime PaO2 |
| Gambirini et al. [10] | Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters | Journal of Endocrinological Investigation | 2003 | Cross-sectional study | Both free and total testosterone levels were lower in patients with OSA and obesity compared to those with obesity alone |
| Perimenis et al. [11] | Erectile dysfunction in men with obstructive sleep apnea syndrome: a randomized study of the efficacy of sildenafil and continuous positive airway pressure | International Journal of Impotence Research | 2004 | RCT | 30 men with OSA and ED divided equally into groups treated with sildenafil vs. CPAP for 12 weeks; successful intercourse 54% vs. 24%; IIEF score increased by 62% vs. 26%; overall satisfaction 53% vs. 20% |
| Taskin et al. [12] | Erectile dysfunction in severe sleep apnea patients and response to CPAP | International Journal of Impotence Research | 2009 | RCT | CPAP (n = 17) vs. SSRI (n = 15) for 1 month; IIEF score increased by 22% vs. 8% |
| Khafagy et al. [13] | Treatment of obstructive sleep apnoea as a therapeutic modality for associated erectile dysfunction | International Journal of Clinical Practice | 2012 | Prospective study | 80 patients with OSA and ED had CPAP for 3 months. Following CPAP, the reduction in patients of all severities of ED with 23% of patients now classified as normal; nocturnal penile rigidity was also shown to be significantly increased with a rise in patients with >80% rigidity from 14% to 40% |
| Pastore et al. [14] | Severe obstructive sleep apnoea syndrome and erectile dysfunction: a prospective randomised study to compare sildenafil vs. nasal continuous positive airway pressure | International Journal of Clinical Practice | 2014 | RCT | CPAP (n = 41) vs. sildenafil (n = 41) for 3 months; successful intercourse 30% vs. 58%; post-intervention IIEF 58% vs. 135% |
| Knapp et al. [15] | Effect of continuous positive airway pressure therapy on sexual function and serum testosterone in males with type 2 diabetes and obstructive sleep apnoea | Clinical Endocrinology | 2014 | Prospective study | 27 patients with T2DM and OSA completed 3 months of CPAP. 84% had some form of ED. No change in total or free testosterone levels or the SHIM score |
| Husnu et al. [16] | Obstructive sleep apnea syndrome and erectile dysfunction: does long term continuous positive airway pressure therapy improve erections? | African Health Sciences | 2015 | Prospective study | 28 patients with OSA underwent CPAP for 3 months. IIEF score was found to increase by 26% |
| Li et al. [17] | Efficacy of nasal continuous positive airway pressure on patients with OSA with erectile dysfunction and low sex hormone levels | Respiratory Medicine | 2016 | Prospective study | 47% of OSA patients also had ED. ED was more common with greater severity of OSA, with 24%, 48%, and 60% in mild, moderate, and severe OSA, respectively. Severe OSA (n = 32) treated with CPAP for 1 month. Improvement in IIEF scores (35%), FSH levels (4.8–11.7 IU/L), and testosterone levels (4.5–12.1 nmol/L) following CPAP |
| Zhang et al. [18] | Erectile Dysfunction and Sexual Hormone Levels in Men With Obstructive Sleep Apnea: Efficacy of Continuous Positive Airway Pressure | Archives of Sexual Behaviour | 2016 | Prospective study | 207 men stratified by OSA severity; 3 months of CPAP; severe OSA patients had lower testosterone (14.06) vs. simple snoring group (17.02). CPAP did not significantly change serum testosterone levels |
| Taken et al. [19] | Erectile dysfunction is a marker for obstructive sleep apnea | The Aging Male | 2016 | Cross-sectional study | 68% of men with OSA suffered with concomitant ED. |
| Kalejaiye et al. [20] | Sleep disorders in patients with erectile dysfunction | BJU International | 2017 | Prospective study | 82% of men with OSA suffered with severe ED, of which 11 had already undergone penile prosthesis insertion |
| Melehan et al. [21] | Randomized Trial of CPAP and Vardenafil on Erectile and Arterial Function in Men With Obstructive Sleep Apnea and Erectile Dysfunction | Journal of Clinical Endocrinology and Metabolism | 2018 | RCT | CPAP vs. PDE5i for 12 weeks; insignificant improvement in IIEF scores (11%) with CPAP; CPAP was found to increase the number of nocturnal erection (2.7–4.1). Arterial stiffness was shown to improve with CPAP (13.7–7.3). No change in testosterone, LH, and FSH with either CPAP or PDE5i |
| Spitzer et al. [22] | Sildenafil increases serum testosterone levels by a direct action on the testes | Andrology | 2018 | Prospective study | Administration of an optimized dose of sildenafil was associated with mean increases of 3.6 nmol/L (103 ng/dL; p < 0.001) and 110 pmol/L (31.7 pg/mL; p < 0.001) in total and free testosterone levels, respectively |
| Grewe et al. [23] | Effects of CPAP treatment on sex hormone levels in patients with obstructive sleep apnoea – data from two randomized, controlled trials | Sleep and Control of Breathing | 2019 | RCT | Continue CPAP (n = 35) or withdraw CPAP (n = 35) for 2 weeks; CPAP withdrawal increased DHEA alone (0.17–1.35) whilst the other hormones remained unaffected |
| Schulz et al. [24] | CPAP therapy improves erectile function in patients with severe obstructive sleep apnea | Sleep Medicine | 2019 | Prospective study | 68% of men suffering with severe OSA also suffered with ED (IIEF score <21) |
| Author . | Title . | Journal . | Year of publication . | Type of study . | Findings . |
|---|---|---|---|---|---|
| Schneider et al. [7] | Influence of testosterone on breathing during sleep | Journal of Applied Physiology | 1987 | Prospective study | Both apnoeas and hypopnoeas increased significantly when hypogonadal men were treated with testosterone replacement |
| Granata et al. [8] | Relationship between sleep-related erections and testosterone levels in men | Journal of Andrology | 1997 | Prospective study | Serum testosterone levels for nocturnal erections were 200 ng/dL |
| Fanfulla et al. [9] | Erectile Dysfunction in Men with Obstructive Sleep Apnea: An Early Sign of Nerve Involvement | Sleep | 2000 | Prospective study | 68% of men with OSA had an altered BCR. These patients had higher AHI, higher percentage of sleep SaO2 <90, and lower daytime PaO2 |
| Gambirini et al. [10] | Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters | Journal of Endocrinological Investigation | 2003 | Cross-sectional study | Both free and total testosterone levels were lower in patients with OSA and obesity compared to those with obesity alone |
| Perimenis et al. [11] | Erectile dysfunction in men with obstructive sleep apnea syndrome: a randomized study of the efficacy of sildenafil and continuous positive airway pressure | International Journal of Impotence Research | 2004 | RCT | 30 men with OSA and ED divided equally into groups treated with sildenafil vs. CPAP for 12 weeks; successful intercourse 54% vs. 24%; IIEF score increased by 62% vs. 26%; overall satisfaction 53% vs. 20% |
| Taskin et al. [12] | Erectile dysfunction in severe sleep apnea patients and response to CPAP | International Journal of Impotence Research | 2009 | RCT | CPAP (n = 17) vs. SSRI (n = 15) for 1 month; IIEF score increased by 22% vs. 8% |
| Khafagy et al. [13] | Treatment of obstructive sleep apnoea as a therapeutic modality for associated erectile dysfunction | International Journal of Clinical Practice | 2012 | Prospective study | 80 patients with OSA and ED had CPAP for 3 months. Following CPAP, the reduction in patients of all severities of ED with 23% of patients now classified as normal; nocturnal penile rigidity was also shown to be significantly increased with a rise in patients with >80% rigidity from 14% to 40% |
| Pastore et al. [14] | Severe obstructive sleep apnoea syndrome and erectile dysfunction: a prospective randomised study to compare sildenafil vs. nasal continuous positive airway pressure | International Journal of Clinical Practice | 2014 | RCT | CPAP (n = 41) vs. sildenafil (n = 41) for 3 months; successful intercourse 30% vs. 58%; post-intervention IIEF 58% vs. 135% |
| Knapp et al. [15] | Effect of continuous positive airway pressure therapy on sexual function and serum testosterone in males with type 2 diabetes and obstructive sleep apnoea | Clinical Endocrinology | 2014 | Prospective study | 27 patients with T2DM and OSA completed 3 months of CPAP. 84% had some form of ED. No change in total or free testosterone levels or the SHIM score |
| Husnu et al. [16] | Obstructive sleep apnea syndrome and erectile dysfunction: does long term continuous positive airway pressure therapy improve erections? | African Health Sciences | 2015 | Prospective study | 28 patients with OSA underwent CPAP for 3 months. IIEF score was found to increase by 26% |
| Li et al. [17] | Efficacy of nasal continuous positive airway pressure on patients with OSA with erectile dysfunction and low sex hormone levels | Respiratory Medicine | 2016 | Prospective study | 47% of OSA patients also had ED. ED was more common with greater severity of OSA, with 24%, 48%, and 60% in mild, moderate, and severe OSA, respectively. Severe OSA (n = 32) treated with CPAP for 1 month. Improvement in IIEF scores (35%), FSH levels (4.8–11.7 IU/L), and testosterone levels (4.5–12.1 nmol/L) following CPAP |
| Zhang et al. [18] | Erectile Dysfunction and Sexual Hormone Levels in Men With Obstructive Sleep Apnea: Efficacy of Continuous Positive Airway Pressure | Archives of Sexual Behaviour | 2016 | Prospective study | 207 men stratified by OSA severity; 3 months of CPAP; severe OSA patients had lower testosterone (14.06) vs. simple snoring group (17.02). CPAP did not significantly change serum testosterone levels |
| Taken et al. [19] | Erectile dysfunction is a marker for obstructive sleep apnea | The Aging Male | 2016 | Cross-sectional study | 68% of men with OSA suffered with concomitant ED. |
| Kalejaiye et al. [20] | Sleep disorders in patients with erectile dysfunction | BJU International | 2017 | Prospective study | 82% of men with OSA suffered with severe ED, of which 11 had already undergone penile prosthesis insertion |
| Melehan et al. [21] | Randomized Trial of CPAP and Vardenafil on Erectile and Arterial Function in Men With Obstructive Sleep Apnea and Erectile Dysfunction | Journal of Clinical Endocrinology and Metabolism | 2018 | RCT | CPAP vs. PDE5i for 12 weeks; insignificant improvement in IIEF scores (11%) with CPAP; CPAP was found to increase the number of nocturnal erection (2.7–4.1). Arterial stiffness was shown to improve with CPAP (13.7–7.3). No change in testosterone, LH, and FSH with either CPAP or PDE5i |
| Spitzer et al. [22] | Sildenafil increases serum testosterone levels by a direct action on the testes | Andrology | 2018 | Prospective study | Administration of an optimized dose of sildenafil was associated with mean increases of 3.6 nmol/L (103 ng/dL; p < 0.001) and 110 pmol/L (31.7 pg/mL; p < 0.001) in total and free testosterone levels, respectively |
| Grewe et al. [23] | Effects of CPAP treatment on sex hormone levels in patients with obstructive sleep apnoea – data from two randomized, controlled trials | Sleep and Control of Breathing | 2019 | RCT | Continue CPAP (n = 35) or withdraw CPAP (n = 35) for 2 weeks; CPAP withdrawal increased DHEA alone (0.17–1.35) whilst the other hormones remained unaffected |
| Schulz et al. [24] | CPAP therapy improves erectile function in patients with severe obstructive sleep apnea | Sleep Medicine | 2019 | Prospective study | 68% of men suffering with severe OSA also suffered with ED (IIEF score <21) |
Data Synthesis
A narrative synthesis was conducted for all included studies. Due to the studies included in the systematic review differing in terms of study design, methods, and population size, it was decided a narrative synthesis was most appropriate to analyse the evidence from these heterogenous studies [25].
The narrative synthesis was conducted by ZAS who conducted the data extraction and analysis. The evidence was summarized and common theses were identified. The synthesis process was reviewed by all authors and final common themes were determined after mutual agreement.
Results
Study Selection
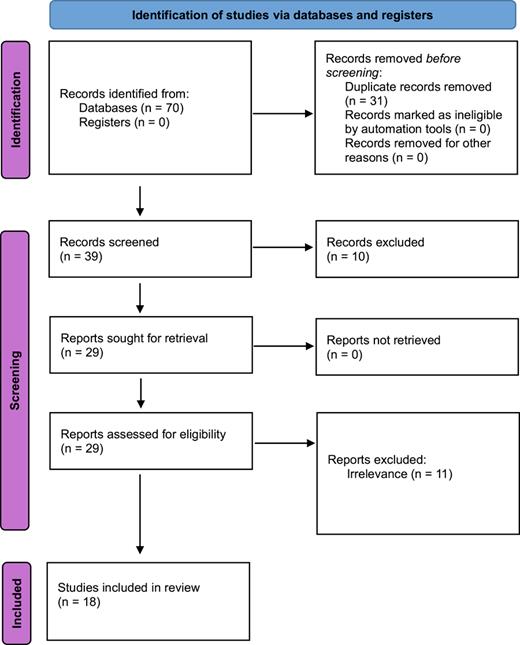
The database search revealed 70 studies that were potentially relevant for this systematic review. Following abstract review, 31 studies were found to be duplicated resulting in 39 remaining studies. Of these studies, 10 were considered irrelevant from abstract review. The remaining 29 full-text articles were reviewed and a further 11 studies excluded due to irrelevance. The final number of relevant studies was 18 comprising 5 randomized controlled trials, 11 prospective studies, and 2 cross-sectional studies (Fig. 1).
PRISMA 2020 flow diagram for identification of studies via databases.
Prevalence of ED in Patients with OSA
There have been several studies that have documented the link between ED and OSA. Schulz et al. [24] studied 94 men with severe OSA (AHI >30/h of sleep. They found that in this population 68% also had ED (IIEF-5 score <21). Fanfulla et al. [9] noted that 17 out of 25 OSA patients (68%) in their study demonstrated an altered bulbocavernosus reflex (BCR) with 11 out of 17 (44%) of these patients having a reduced amplitude and prolonged latency period of BCR. The patients with altered BCR all had a higher AHI, greater proportion of sleep with oxygen saturations <90%, and lower daytime Pa02. Taken et al. [19] noted a similar proportion of patients (63%) with OSA suffering with commitment ED from a cohort of 55 patients. Kalejaiye et al. [20] found that 82% of men with OSA in their study suffered with severe ED, of which 11 had already undergone penile prosthesis insertion.
Relationship between OSA and Hormonal Physiology Resulting in ED
There is evidence to support a possible association of lower testosterone levels as a possible cause of OSA in ED. Under the control of luteinizing hormone and follicle stimulating hormone, the synthesis of testosterone is regulated via the hypothalamic pituitary testicular axis [26, 27]. Studies have shown that patients with OSA have lower serum testosterone levels [17] and an increasing apnoea hypoxia index results in a lower serum testosterone level [18]. When testosterone has been found to be <6.9 nmol/L, this resulted in fewer nocturnal erections [8]. Studies have also shown that testosterone levels were lower in patients with OSA and obesity than in patients with obesity alone [10]. On the contrary, it has also been shown that treatment of low testosterone with supplements, in patients with OSA, can exacerbate and worsen the severity of OSA, thus implying testosterones role in the pathophysiology of OSA [7].
Effectiveness of PDE5i in the Treatment of ED in Patients with OSA
PDE5i are commonly used in the treatment of ED. There has been increased interest into its effectiveness in patients with OSA for a number of reasons. Primarily, OSA is associated with low testosterone and PDE5i are theorized to improve testosterone levels in a small but significant margin [22]. Mean improvements of 3.6 nmol/L were seen in total serum testosterone levels, thought to be due to direct effects of sildenafil on the testes. It is hypothesized that PDE5i improve endothelial function and thus testicular blood flow. This can theoretically positively impact Leydig cell steroidogenesis [22]. A study by Melehan et al. [21] showed that vardenafil used in men with OSA and ED did not significantly improve testosterone levels, erectile function, or number of nocturnal erections. It did however report an improvement in overall sexual satisfaction and self-esteem. This contrasts with studies by Pastore et al. and Perimenis et al. where patients were treated with either sildenafil or CPAP and found to have improved rate of sexual intercourse, IIEF scores, and overall satisfaction.
Evidence for Treatment of OSA Benefitting ED
There has been interest in the effect of treating OSA with CPAP on the severity of ED. Perimenis et al. [11] compared the efficacy of sildenafil and CPAP in men with both OSA and ED. They divided 30 men into 2 groups of 15 each treated with either sildenafil or CPAP for 12 weeks. The two groups were matched for age (56.4 vs. 55.7), severity of ED (IIEF 23.3 vs. 21.7), and OSA (AHI 7.4 vs. 7.3). Sildenafil produced a 54% success rate for attempted intercourse, whilst 24% of attempts were successful in patients receiving CPAP. In addition, both sildenafil and CPAP increased the mean IIEF score from 23.3 to 37.7 (62% improvement) vs. 21.7 to 27.3 (26% improvement), respectively. Whilst a greater proportion of patients in the sildenafil group were satisfied with treatment (53%) compared to the CPAP group (20%), this study revealed the positive impact of CPAP on sexual function.
A similar study by Pastore et al. [14] studied 82 patients with OSA and ED divided into 2 equal groups treated with either sildenafil or CPAP for 3 months. Once again, groups were matched for age (47.4 vs. 48.6), severity of ED (IIEF 7.8 vs. 7.4), and OSA (AHI 46.9 vs. 47.3). Those treated with sildenafil were found to have successful intercourse on 58% of occasions whilst this was less effective in the CPAP group with only 30% success with intercourse. When IIEF scores were measured post-intervention, they were found to be higher in the sildenafil group (18.3, 135% improvement) vs. the CPAP group (11.7, 58% improvement).
Taskin et al. [12] also investigated the effect of CPAP on ED in patients with OSA. They divided 32 patients into 2 groups of 17 and 15, treated with either CPAP or SSRI antidepressants, respectively, for 1 month. Within the CPAP group, all the patients were confirmed as severe OSA with mean AHI 35 +/− 19 and their mean IIEF was 15.7. They found that CPAP increased mean IIEF score from 15.7 to 19.1 (22% improvement) vs. 13.1 to 14.1 (8% improvement) caused by SSRI therapy. It was concluded that CPAP was effective in improving sexual performance in this cohort of patients.
Husnu et al. [16] studied 90 patients, which were divided into 3 groups based on their severity of OSA. Group 1 included 28 patients without OSA (AHI <5), group 2 included 29 patients with mild/moderate OSA (AHI 5–29), and group 3 included 33 patients with severe OSA (AHI >30). Only 28 participants from group 2 (n = 11) and group 3 (n = 17) underwent CPAP for 3 months. It was noted that mean IIEF score was found to increase 16.6 to 20.9 (26% improvement) in these patients, with no significant difference seen despite difference in severity of OSA.
Li et al. [17] investigated 153 patients with OSA and 60 healthy controls. IIEF scores determined 47.1% of all OSA patients also suffered with ED (IIEF <21). This was found to be more common with increasing severity of OSA, with 24%, 48%, and 60% in mild (AHI 5–15), moderate (AHI 15–30), and severe OSA (AHI >30), respectively. Only 13% of control patients suffered with ED. They then further dived the patients into 2 groups, 1 with OSA and ED (n = 72) and 1 with OSA without ED (n = 81). As mentioned, the OSA/ED group had a greater severity of OSA (AHI 56.8) compared to the OSA alone group (AHI 22.9). In addition, the OSA/ED group was also found to have lower serum and testosterone levels compared to the OSA alone group. In the OSA/ED group, 32 patients had severe OSA and were subjected to CPAP for 1 month. They noted a significant improvement in IIEF scores (14.2–19.2, 35% improvement), FSH levels (4.8–11.7 IU/L), and testosterone levels (4.5–12.1 nmol/L) following CPAP. The conclusion was made that ED was more prevalent in patients with OSA and these patients often had decreased sex hormones, both of which showed some improvement with CPAP therapy.
Grewe et al. [23] studied the effect of CPAP withdrawal on sex hormone levels in patients with OSA. They randomized 70 patients into 2 groups that would either continue CPAP or have their CPAP withdrawn for 2 weeks. They measured the following hormones pre- and post-intervention: androstenedione, DHEA, 17-hydroxyprogesterone, progesterone, testosterone, pregnenolone, FSH, and LH. They found that CPAP withdrawal increased DHEA alone (0.17–1.35) whilst the other hormones remained unaffected.
Melehan et al. [21] investigated the effect of CPAP vs. PDE-5 inhibitor on erectile and arterial function in 61 patients with severe OSA (AHI >30) and ED. These patients were randomized into group 1 (CPAP + PDE5i), group 2 (CPAP + placebo), group 3 (sham + PDE5i), and group 4 (sham + placebo) and studied for 12 weeks. They noted an improvement in IIEF scores (39.0–43.3, 11% improvement) with CPAP; however, the difference in improvement compared with sham was not statistically significant. When PDE5i was compared with placebo, the difference in improvement of IIEF scores (7.9) was also not found to be statistically significant, which the authors suggested may be due to OSA patients being partly resistant to low dose continuous PDE5 inhibitor use. When nocturnal erections were studied, CPAP was found to increase the number of nocturnal erection (2.7–4.1) compared to sham (2.5–2.4); however, PDE5i did (3.0–3.4) not when compared to placebo (2.2–3.2). Arterial stiffness was shown to improve with CPAP (13.7–7.3) compared to sham (12.0–11.3). PDE5i did not significantly reduce arterial stiffness when compared to placebo when measured using pulse wave analysis and peripheral arterial tonometry. This study did not find any change in testosterone, LH, and FSH with either CPAP or PDE5i.
Knapp et al. [15] investigated the role of CPAP on ED in patients with T2DM and OSA. In their study, 27 patients completed the 3-month trial. In this cohort, 84% were found to have some degree of ED (severe ED, n = 12, moderate ED, n = 2, moderate-mild ED, n = 5, mild ED, n = 2). They found no change in total or free testosterone levels or the sexual health inventory for men (SHIM) score. They concluded CPAP did not directly benefit gonadal or sexual function.
Khafagy et al. [13] subjected 80 patients with OSA and ED to 3 months of CPAP therapy and measured erectile function using both IIEF scores and nocturnal penile rigidity measurement. They found that pre-treatment their subjects suffered with mild (54%), moderate (30%), and severe (16%) ED according to IIEF scores. Following CPAP, there was reduction in patients of all severities of ED (mild 11%, moderate 19%, severe 48%), with 23% of patients now classified as normal. Nocturnal penile rigidity was also shown to be significantly increased with a rise in patients with >80% rigidity from 14% to 40%.
Discussion
There has long been a proposed link between OSA and ED. Our study has found evidence to support this with included studies unanimously reporting a significant proportion of men with OSA suffering with ED. The studies defined ED using a variety of measures including IIEF scores and measuring BCR using electrophysiological testing. Along with demonstrating that ED was present in patients with OSA between 68 and 82% [9, 19, 20, 24], the studies also demonstrated that the greater the severity of OSA the worse the degree of ED [9]. Husnu et al. [16] were able to demonstrate that despite the differing severities of OSA, the effect of CPAP on improving erectile function was unaffected.
This relationship has led to the hypothesis that perhaps treating OSA using CPAP may result in improvement, if not complete resolution, of ED. A number of studies have investigated this hypothesis and there have been studies that have reported improvements in ED when their OSA was treated with CPAP for as little as 1 month. Taskin et al. [12] were able to demonstrate a significant improvement in IIEF score with 1 month of CPAP. Similarly, Li et al. [17] also reported significant improvements in IIEF score following 1 month of CPAP. They were also able to demonstrate that this coincided with an increase in FSH and testosterone levels, thus postulating that sex hormones may be deficient in men suffering with ED and OSA and that CPAP treatment helps to improve hormone levels and thereby improves the degree of ED. However, further studies [15, 21, 23] studied the hormone profile of patients subjected to CPAP and were unable to find any significant change in sex hormones other than a rise in DHEA.
Other studies treated patients with CPAP for longer periods such as 3 months [13, 16] and also reported a benefit for ED. One of these studies [13] even found 23% of their participants to no longer suffer with ED according to IIEF scores following 3 months of CPAP.
There have also been studies that have shown some benefit with CPAP; however when compared to sildenafil, the improvements were not as impressive [11, 14]. Both of these studies treated men with either CPAP or sildenafil for 3 months. Perimenis et al. and Pastore et al. were able to show an improvement in both IIEF score and percentage successful intercourse following CPAP therapy; however, these were less significant than the results gained following sildenafil treatment. The participants also reported much greater treatment satisfaction in the sildenafil cohort.
Finally, there are also studies that have shown no statistical improvement in sexual function following treatment with CPAP. Both Melehan et al. [21] and Knapp et al. [15] were unable to show any significant improvement in sexual function according to IIEF and SHIM scores, respectively, after 3 months of CPAP treatment.
There has been a relative lack of studies investigating the possible link between OSA and ED. Although some interest around treatment has been present, there appears to be a lack of randomized controlled trials and a lack of consistency in the tools used to measure the degree of ED. Furthermore, as patients suffering with OSA and ED usually belong to a cohort of individuals that often suffer with a host of other co-morbidities as part of the metabolic syndrome, this can make it difficult to control for these confounding variables in order to be certain of a true link between OSA and ED and the responses to treatment.
Strengths and Limitations
This study is the most updated comprehensive review of the literature summarizing the evidence linking ED and OSA, the pathogenesis behind this, and the potential treatment options possible. This study has performed a robust literature search and quality control was conducted by all authors to ensure selection bias was minimized.
It is acknowledged that this review contains studies of different designs and protocols, from which it can be difficult to compare the outcomes. It is also recognized that given the relatively recent interest in this topic, there are few papers in the literature studying the link between ED and OSA. This has led to some aspects of research where validity cannot be extensively commented on due to the lack of evidence of reproducible data.
Conclusion
There does appear to be an association between the presence of OSA and ED. The literature has reported up to 82% prevalence of ED in patients with OSA. Studies investigating the role of CPAP in treating ED in this cohort of patients has shown results varying from no significant effect to complete resolution of ED. Further randomized controlled trials to evaluate the efficacy of CPAP on ED in patients with OSA need to be conducted. These studies should aim to evaluate ED using a multitude of tools (IIEF, SHIM, BCR, penile rigidity, sex hormone profile) and have stricter inclusion criteria to avoid confounding co-morbidities. Until more robust evidence is available, CPAP is a safe treatment likely to improve symptoms in individuals with OSA. However, the degree to which it may affect ED symptoms is not yet clear, but what appears clear is that it does not negatively impact on erectile function and is most likely to offer some degree of improvement.
Statement of Ethics
This study was exclusively based on published literature and therefore ethical approval and written informed consent were not required in accordance with local/national guidelines.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
The authors have no funding to declare.
Author Contributions
All authors (Z.A.S., D.P.M., A.S., I.P., V.M.) were involved in performing the literature review to select relevant papers for inclusion in the systematic review. Z.A.S. wrote the initial draft with A.S. and V.M. contributing to produce the final draft for submission.
Data Availability Statement
All data generated or analysed during this study are included in this article and its online supplementary material files. Further enquiries can be directed to the corresponding author.