Abstract
Background: This work had its origins in the 1990s, when women having collagen-creating midurethral slings for stress urinary incontinence (SUI) and uterosacral slings for uterine prolapse began reporting cure or improvement in co-occurring fecal incontinence, obstructive defecation, and chronic pelvic pain. Summary: We briefly describe anatomical etiopathogenesis to explain how the same collagen-creating ligament repair system using a common ligament-based diagnostic system, can treat pelvic symptoms from 3 disciplines: Urology, Gynecology, Coloproctology. Collagen-induced laxity in ligaments and vagina diminishes contractile forces required by pelvic muscles to close urethra and anus for continence, open them for evacuation, and stretch the bladder base and rectum like a trampoline to prevent stretch receptors prematurely activating micturition and defecation reflexes. These are perceived cortically as bladder or fecal “urge to go.” The pictorial algorithm summarizes common ligament pathogeneses for prolapse/bladder/bowel/pain dysfunctions which can be confirmed by mechanical support of PUL for relief of urine loss on coughing, and uterosacral ligaments (USL) for relief of urge and chronic pelvic pain. The same minimally invasive ligament repairs used for SUI, prolapse, pain/bladder dysfunctions were demonstrated by X-ray defecography controlled studies to cure fecal incontinence, obstructive defecation, anterior rectal wall intussusception and descending perineal syndrome (as shown in 16 case managements in 3 disciplines; video: https://youtu.be/a6jJQXDx71U?si=MLdo3Yq9kAZ82RVb). Key Messages: Symptom relief can be achieved using standard operations which repair PUL or USL even with minimal prolapse. Whether the surgery is done laparoscopically or vaginally is of little consequence, as the same structure is repaired.
Background
This work had its origins in the 1990s, when women having collagen-creating midurethral slings for stress urinary incontinence (SUI) and uterosacral slings for uterine prolapse began reporting cure or improvement in co-occurring fecal incontinence and obstructive defecation and chronic pelvic pain (CPP) [1]. Also cured were symptoms of urge, nocturia and CPP, and later, as demonstrated by X-ray defecography controlled studies, fecal incontinence, obstructive defecation and anterior rectal wall intussusception. These reports inspired clinical research investigations by many different authors.
Introduction
The developed world population is aging rapidly [2]. Associated with aging are pelvic organ prolapse and incontinence conditions which increase with age [3]. The result is poor quality of life, escalating community and government health costs, and pressure on nursing homes.
The prevalence of urinary incontinence approaches 70% in care homes [3]; nocturia in women aged 60–70 years is a problem in 11%–50% and prevalence rises to 80%–90% at 80 years [4]. Hip fractures can occur in 4.6% of nocturic women, occurrence increasing with age [4]. Inability to empty can occur in up to 59% of women [5]. In a survey of women older than 40 years of age, the prevalence of fecal incontinence was 24% [6].
Despite the knowledge that organ prolapse, bladder/bowel/pain dysfunctions are known to co-occur, patients are still being directed to three disciplines, urology, gynecology, coloproctology. All have different nomenclature, diagnostic protocols and treatments.
That there is a treatment crisis is self-evident. The prevalence of bladder, bowel, CPP, and prolapse in an aging population has been estimated as up to 25% of the four billion women in the world. Other than surgical cure of SUI by the midurethral sling, which itself is based on the Integral Theory Paradigm (ITP) [1], it is generally accepted that the pathogenesis of bladder/bowel/pain dysfunctions is largely unknown, and no surgical cure is possible. Because of major complications, large mesh surgeries have been banned in many countries, only to see them surface with abdominal implantation in sacrocolpopexies and rectopexies. Unsurprisingly, mesh-related problems are being increasingly reported with such surgeries.
First-line symptom treatments such as anticholinergics for overactive bladder (OAB) are hardly effective [7], have bothersome complications, and can exacerbate dementia syndromes [8]. Opioid treatments for CPP can lead to lifetime addiction. Second-line treatments such as sacral nerve stimulation and Onabotulinumtoxin A are expensive [9‒11] and are not easily available for the majority of the one billion women who suffer from these pelvic conditions.
The ITP – A Current Solution to the Problem
The ligament-based system offers a new way forward. It is a different way of thinking. It fits Thomas Kuhn’s description of a new paradigm, “a fundamental change in the basic concepts and experimental practices of a scientific discipline” [12]. In contrast to previous thinking, the ligament-based system aims primarily to treat the pelvic symptoms mentioned above even if prolapse is minimal. This is because even a slightly altered anatomy can cause severe symptoms.
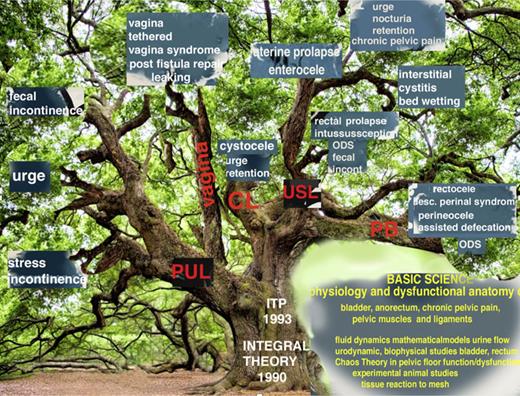
Though this system started as a new operation for SUI, in the past 30 years, it has grown into a parallel universe to historical managements which still prevail. Its scope for cure of bladder/bowel/pain and prolapse dysfunctions is graphically summarized (as shown in Fig. 1).
The Integral Theory Paradigm (ITP) discovery tree. The ITP tree shows the expansion of the ITP between 1990 and 2023 to cure bladder/bowel/pain/prolapse conditions by strengthening ligaments, muscles and restoring elasticity to the vagina by skin grafts by many different physicians. Many conditions in Figure 1, such as interstitial cystitis/BPS, cure of ongoing massive urine loss after successful fistula repair, obstructive defecation syndrome, anterior rectal wall intussusception were discovered by observation and testing following ligament repair according to the ITP. Some such as cure of day/night enuresis and the latest urethral ligament plication operation were by direct testing the ITP’s predictions (see 2024 Integral Theory Update: https://atm.amegroups.org/issue/view/1400). BPS, bladder pain syndrome.
The Integral Theory Paradigm (ITP) discovery tree. The ITP tree shows the expansion of the ITP between 1990 and 2023 to cure bladder/bowel/pain/prolapse conditions by strengthening ligaments, muscles and restoring elasticity to the vagina by skin grafts by many different physicians. Many conditions in Figure 1, such as interstitial cystitis/BPS, cure of ongoing massive urine loss after successful fistula repair, obstructive defecation syndrome, anterior rectal wall intussusception were discovered by observation and testing following ligament repair according to the ITP. Some such as cure of day/night enuresis and the latest urethral ligament plication operation were by direct testing the ITP’s predictions (see 2024 Integral Theory Update: https://atm.amegroups.org/issue/view/1400). BPS, bladder pain syndrome.
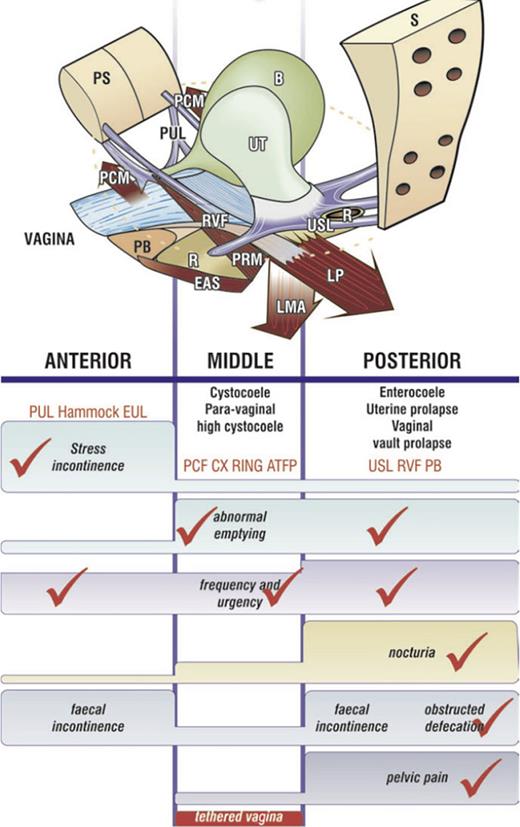
In its present form, this method can be condensed as follows: “pelvic organ prolapse, CPP, bladder and bowel dysfunctions are mainly caused, for different reasons, by laxity in 4 main suspensory ligaments, a consequence of altered collagen/elastin” [13]. The same collagen-based system is applied to treat widely varying conditions such as prolapse/bladder/bowel/pain dysfunctions, repairing damaged ligaments in three zones of the vagina (Figure 2) [13‒50] (as shown in video link diagnosis: https://youtu.be/FlBX0iQIE_s?si=XPFV0wAzXZA-mJCg).
The pictorial diagnostic algorithm represents three zones of connective tissue causation. Positive symptoms (even “sometimes”) are ticked in each column where they occur, and the causative prolapse and ligaments emerge. The prolapses in the algorithm correlate with ligament damage in the columns. Symptom groupings in the 3 columns help to deduce which ligaments cause which symptoms and serve as a guide to surgical repair of the ligaments. Pelvic pain and nocturia are only caused by uterosacral ligament (USL) laxity. Severe stress incontinence is caused mainly by pubourethral ligament (PUL) laxity. Hammock, suburethral vaginal hammock; EUL, external urethral ligament attached to the external urethral meatus; CX ring represents cardinal ligament insertion onto the anterior part of the cervical ring; PCF, pubocervical fascia; RVF, rectovaginal fascia; PB, perineal body; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; EAS, external anal sphincter; PRM, puborectalis muscle; PCM, pubococcygeus muscle; R, rectum; UT, uterus; B, bladder; PS, pubic symphysis; S, sacrum.
The pictorial diagnostic algorithm represents three zones of connective tissue causation. Positive symptoms (even “sometimes”) are ticked in each column where they occur, and the causative prolapse and ligaments emerge. The prolapses in the algorithm correlate with ligament damage in the columns. Symptom groupings in the 3 columns help to deduce which ligaments cause which symptoms and serve as a guide to surgical repair of the ligaments. Pelvic pain and nocturia are only caused by uterosacral ligament (USL) laxity. Severe stress incontinence is caused mainly by pubourethral ligament (PUL) laxity. Hammock, suburethral vaginal hammock; EUL, external urethral ligament attached to the external urethral meatus; CX ring represents cardinal ligament insertion onto the anterior part of the cervical ring; PCF, pubocervical fascia; RVF, rectovaginal fascia; PB, perineal body; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; EAS, external anal sphincter; PRM, puborectalis muscle; PCM, pubococcygeus muscle; R, rectum; UT, uterus; B, bladder; PS, pubic symphysis; S, sacrum.
Surgery is guided by the diagnostic algorithm (as shown in Fig. 2), which summarizes the ITP. It shows the direct association between symptoms, prolapse, and ligament damage, and so acts as an anatomical guideline for management. The video link examples 16 cases from all 3 disciplines, Urology, Gynecology, Coloproctology, managed by the same ligament-based diagnostic and surgical methodology: https://youtu.be/a6jJQXDx71U?si=MLdo3Yq9kAZ82RAVb (see also online suppl. Video at https://doi.org/10.1159/000545665).
A Singular Holistic System for Diagnosis and Management
The aim of this short narrative review was to present a singular s holistic system for diagnosis and management of the following conditions: organ prolapse, bladder/bowel/pain dysfunctions. We revisited known studies by 45 different authors in a recent publication based on this system (as shown in the Integral Theory Update link: https://atm.amegroups.org/issue/view/1400). We analyzed these and other prior studies, with regard to the role of lax or weak ligaments in pathophysiology, pathogenesis and reported cure of the above conditions.
A Paradigm Shift
The thrust of this work is that the ITP is a paradigm shift. In his book, The Structure of Scientific Revolutions, Kuhn [12] defined a paradigm shift as “a fundamental change in the way a scientific discipline thinks and practices: [12]. The following sections describe important discoveries of changes in thinking.
Three Key 1990 Discoveries
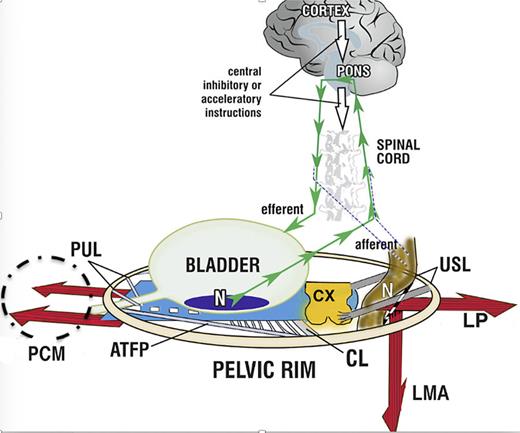
Three key discoveries in 1990 lie at the core of almost all discoveries attributed to the ITP (as shown in Fig. 1). The first key discovery, validated by ultrasound and X-ray data, was that normal bladder control is achieved from outside the organ, from 3 oppositely acting pelvic muscles acting against two main ligaments, pubourethral (PUL) and uterosacral (USL) [1] (https://www.youtube.com/watch?v=GkK5V6LvC3w&t=7s).
With reference to Figure 3, working under central nervous system control, these oppositely acting muscles (variously) close the urethra for continence, open it for micturition, and stretch the vagina and bladder base like a trampoline to prevent premature evacuation of the bladder (“urge incontinence” or “OAB”) [1]. In the context of the original bladder theory, the 3 elements of OAB, urge, frequency, nocturia, are three related phenotypes of a prematurely activated, but otherwise normal micturition [1]. A similar functional anatomy holds for the anorectum [13].
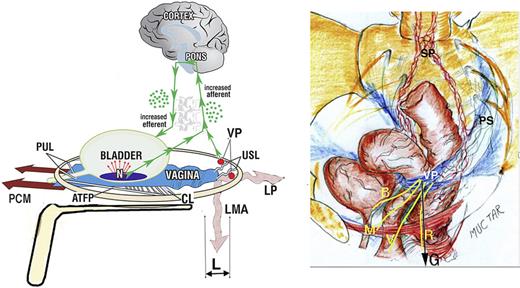
The binary cortico/peripheral control systems of bladder and bowel are virtually identical. Afferent impulses from stretch receptors “N” in the bladder and bowel proceed to the brain which interprets them as “fullness.” By reflexly stretching bladder and bowel bidirectionally (large arrows), the muscles tension the underlying supports of the stretch receptors “N” of the bladder or rectum to support their contents; this prevents them from firing off emptying impulses prematurely, thereby controlling inappropriate activation of the micturition and defecation reflexes, sensed by the cortex as “urge to go.” If convenient to empty, the closure reflex shuts down, and the emptying reflexes (micturition and defecation) are activated. The posterior walls of the urethra and anorectum are actively pulled open (broken lines) by LP/LMA immediately prior to evacuation. This external opening exponentially decreases resistance to flow, thereby facilitating evacuation. Dysfunction. Anatomical damage to any part of the system may interfere with the binary control of all the above functions. Cortex: facilitatory or inhibitory centers; nerves: afferent or efferent (for example, MS); peripheral: ligament or muscle damage; pressure or inflammation on stretch receptors “N” by cancer, cervical fibroid, bladder or rectal prolapse. Surgical cure. “Repair the structure (ligaments, vagina) and you will restore the function” (1). The diagnostic algorithm (as shown in Fig. 2) indicates which ligaments or fascias may be damaged. USL (as shown in Fig. 5) and PUL (as shown in Fig. 6) can be tested for symptom improvement by mechanical support, thereby predicting a high possibility of cure. PUL, pubourethral ligament; USL, uterosacral ligament; CL, cardinal ligament; N, bladder base and anorectal stretch receptors; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; EAS, external anal sphincter; PCM, pubococcygeus muscle.
The binary cortico/peripheral control systems of bladder and bowel are virtually identical. Afferent impulses from stretch receptors “N” in the bladder and bowel proceed to the brain which interprets them as “fullness.” By reflexly stretching bladder and bowel bidirectionally (large arrows), the muscles tension the underlying supports of the stretch receptors “N” of the bladder or rectum to support their contents; this prevents them from firing off emptying impulses prematurely, thereby controlling inappropriate activation of the micturition and defecation reflexes, sensed by the cortex as “urge to go.” If convenient to empty, the closure reflex shuts down, and the emptying reflexes (micturition and defecation) are activated. The posterior walls of the urethra and anorectum are actively pulled open (broken lines) by LP/LMA immediately prior to evacuation. This external opening exponentially decreases resistance to flow, thereby facilitating evacuation. Dysfunction. Anatomical damage to any part of the system may interfere with the binary control of all the above functions. Cortex: facilitatory or inhibitory centers; nerves: afferent or efferent (for example, MS); peripheral: ligament or muscle damage; pressure or inflammation on stretch receptors “N” by cancer, cervical fibroid, bladder or rectal prolapse. Surgical cure. “Repair the structure (ligaments, vagina) and you will restore the function” (1). The diagnostic algorithm (as shown in Fig. 2) indicates which ligaments or fascias may be damaged. USL (as shown in Fig. 5) and PUL (as shown in Fig. 6) can be tested for symptom improvement by mechanical support, thereby predicting a high possibility of cure. PUL, pubourethral ligament; USL, uterosacral ligament; CL, cardinal ligament; N, bladder base and anorectal stretch receptors; LP, levator plate; LMA, conjoint longitudinal muscle of the anus; EAS, external anal sphincter; PCM, pubococcygeus muscle.
The second key discovery was that the ligaments, or more specifically, the structural collagen of the ligaments, were the most vulnerable part of the system, as ligaments could be congenitally loose, or become damaged during childbirth and old age [1]. The third key discovery was a new surgical principle for repair of collagen-deficient ligaments, a method for the creation of artificial collagenous neoligaments by harnessing the tissue reaction to precisely implanted tapes [16]. Applied to damaged suspensory ligaments as per the algorithm (Figure 2), these new surgical methods cured or substantially improved the prolapses and symptoms in the algorithm column, with cure rates ranging from 65% to 85% [13‒50].
It has been found that native ligament repairs work reasonably well in premenopausal women for OAB [38] and CPP [47]. However, because collagen breaks down after the menopause, collagen-creating methods such as slings have been found to be more effective over several years in older postmenopausal women [24, 35].
Bladder and Bowel Function and Dysfunction
Control of bladder and bowel is cortical, reflex, binary, almost identical, but they are also subject to voluntary control (as shown in Fig. 3). With reference to Figure 3, if PUL or USL ligaments are weak or loose, dysfunctions may be expressed clinically as stress incontinence on effort (urinary, fecal), inability to evacuate (urinary, fecal), and inability to “hold on” (OAB); urge and frequency (urinary, fecal) are considered to be prematurely activated evacuation reflexes because of inability of the cortical or peripheral mechanisms (control of “N”) to suppress them [1, 13].
Fecal Incontinence Obstructed Defecation, Descending Perineal Syndrome
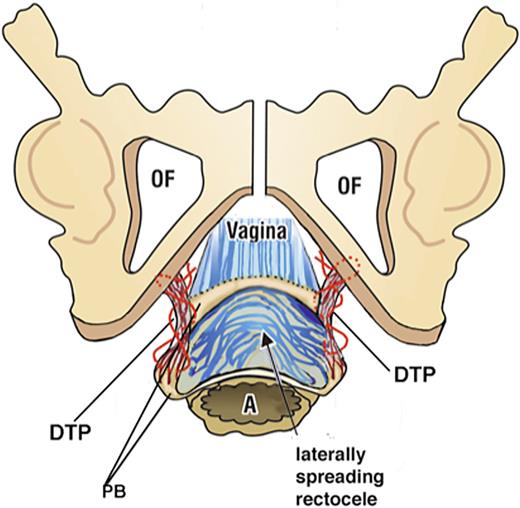
In a pre- and post-operative X-ray defecography controlled study, Abendstein et al. [30] demonstrated that a posterior sling in USLs simultaneously cured fecal incontinence, obstructive defecation, anterior rectal wall intussusception, and CPP. In another study of obstructive defecation, descending perineal syndrome and perineocele, the main cause was found to be elongation of the deep transversus perinei ligament, which was mainly birth-related [18] (as shown in Fig. 4). A ligament-based anorectal theory by Petros and Swash [15] based on 12 experimental studies, which included simultaneous surgical cures for bladder and bowel incontinence was published in 2008 (as shown in the link: https://www.researchgate.net/publication/267778578_The_MusculoElastic_Theory_of_anorectal_function_and_dysfunction).
Anatomy of the descending perineal syndrome. Surgery to locate and repair the DTPs (deep transversus perinei) which are the suspensory ligament of the perineal body requires a much wider dissection. Exposure of a widely laterally displaced perineal body and elongated deep transverse perineal ligament supports is made by a 5-cm full-thickness transverse incision made just inside the hymenal ring. With descending perineal syndrome, the serosa and smooth muscle wall of the rectum are ruptured, and the rectal mucosa is spread to become adherent to the vagina and deep transversus perinei. Careful dissection is required to separate the rectal mucosa. Two to three interrupted sutures close the smooth muscle layer. The DTP is identified and repaired. As the DTP shortens, the perineal bodies fold inward and are gently approximated. The vagina is sutured without excision of tissue (as shown in doi: 10.21037/atm-23-1803). DTP, deep transversus perinei.
Anatomy of the descending perineal syndrome. Surgery to locate and repair the DTPs (deep transversus perinei) which are the suspensory ligament of the perineal body requires a much wider dissection. Exposure of a widely laterally displaced perineal body and elongated deep transverse perineal ligament supports is made by a 5-cm full-thickness transverse incision made just inside the hymenal ring. With descending perineal syndrome, the serosa and smooth muscle wall of the rectum are ruptured, and the rectal mucosa is spread to become adherent to the vagina and deep transversus perinei. Careful dissection is required to separate the rectal mucosa. Two to three interrupted sutures close the smooth muscle layer. The DTP is identified and repaired. As the DTP shortens, the perineal bodies fold inward and are gently approximated. The vagina is sutured without excision of tissue (as shown in doi: 10.21037/atm-23-1803). DTP, deep transversus perinei.
Chronic Pelvic Pain
The discovery of USL laxity as a cause of CPP was published in the German literature in 1938 by Martius [48] and independently in the English literature in 1993 by Peter Petros [47, 49]. In a laparoscopically controlled study, Petros confirmed there was no evident pathogenesis in such patients; he hypothesized that the CPP was a referred pain from the inability of loose USLs to support pelvic visceral plexuses (VP) T11-L2 [47]. Petros [47] found that the pain was generally relieved on lying down, and that a ring pessary also relieved the pain in half the patients. These findings led to the hypothesis (as shown in Fig. 5 right) that the force of gravity “G” stimulated groups of afferent axons within the unsupported VPs to collectively send newly created afferent impulses to the cortex; these impulses were (falsely) interpreted as pain from multiple end organ sites such as vulva, coccyx, lower abdomen, bladder, anus Figure 5.
A “simulated operation” relieves pain and urge by supporting the uterosacral ligaments “USL.” Left image shows 3D view of PUL and USL attachments to the pelvic brim. “L” denotes USL laxity. A gently inserted speculum mechanically supports lax USLs and pelvic visceral nerve plexuses “VP.” The test, if successful, decreases afferent pain and urge impulses; the patient reports lessening or disappearance of pain in multiple sites, for example, B, R, M (right image). Co-occurring urge is also often relieved by speculum support of urothelial stretch receptors “N.” Right image shows 3D view of pelvic organs. VP comprises the sympathetic plexus “SP,” and the parasympathetic plexus “PS.” The yellow lines represent visceral nerves to and from the end organs, M (muscles), V (vagina/vulva), B (bladder), R (rectum) converging on VP, which acts as a relay station on their passage to the spinal cord and brain. G, force of gravity acting on VPs; PCM, pubococcygeus muscle; PUL, pubourethral ligament; LP, levator plate; LMA, conjoint longitudinal muscle of the anus.
A “simulated operation” relieves pain and urge by supporting the uterosacral ligaments “USL.” Left image shows 3D view of PUL and USL attachments to the pelvic brim. “L” denotes USL laxity. A gently inserted speculum mechanically supports lax USLs and pelvic visceral nerve plexuses “VP.” The test, if successful, decreases afferent pain and urge impulses; the patient reports lessening or disappearance of pain in multiple sites, for example, B, R, M (right image). Co-occurring urge is also often relieved by speculum support of urothelial stretch receptors “N.” Right image shows 3D view of pelvic organs. VP comprises the sympathetic plexus “SP,” and the parasympathetic plexus “PS.” The yellow lines represent visceral nerves to and from the end organs, M (muscles), V (vagina/vulva), B (bladder), R (rectum) converging on VP, which acts as a relay station on their passage to the spinal cord and brain. G, force of gravity acting on VPs; PCM, pubococcygeus muscle; PUL, pubourethral ligament; LP, levator plate; LMA, conjoint longitudinal muscle of the anus.
CPP Co-Occurs at Multiple Sites
With reference to Figure 5, afferent visceral nerves, for example, from pelvic muscles “M,” bladder “B,” vagina/vulva “V,” anorectum “R,” independently run to the VPs where they group and synapse. VPs act as a relay station where the visceral nerves continue to the spinal cord and cortex. On the basis that CPP can be relieved on lying down, it is hypothesized that if VPs are unsupported by USLs, the axons can be stimulated by the force of gravity “G” to send off “de novo” impulses to the cortex. These impulses are interpreted as pain arising from the end organs, not from the VPs when they arise. If the pains are relieved by the speculum test, the Bornstein local anesthetic test, the pains at different sites are generally relieved as a group by USL surgery [47, 49].
Testing VP Origins of CPP
The speculum test [51] (as shown in Fig. 5) can relieve CPP by mechanically supporting the USLs and, therefore, VPs. It can also, at the same time, relieve urgency by supporting the urothelial stretch receptors “N,” (as shown in Fig. 5). As such, the speculum test becomes an objective test for USL origins of interstitial cystitis/bladder pain syndrome [27, 37]. Relief of CPP by local anesthetic injection into the USLs at 4 and 8 o’clock, 2 cm from their insertion into the cervix (Bornstein test) [52], is the definitive test for VP origin of CPP. Relief of pain by lying down fits the ITP hypothesis of gravity stimulating VPs unsupported by weak USLs.
Surgical Cure of CPP
Surgical cure of CPP was achieved in 70% at 12 months by native USL repair by Petros [47], in mainly premenopausal women. Cure rates of 80% for CPP have been achieved in postmenopausal women by slings (Table 1) [35] and in other studies [36, 37, 39‒41].
Five-year data from the first cohort of 68 patients 95% confidence intervals commencing in 2009
| Time after TFS . | Cure of prolapse . | Cure of SUI . | Cure of urgency . | Cure of nocturia . | Cure of day time frequency . | Cure of dragging pain . | Cure of dysuria . | Cure of fecal incontinence . |
|---|---|---|---|---|---|---|---|---|
| 12 months | 62/68 | 29/31 | 30/31 | 17/18 | 30/32 | 13/14 | 35/38 | 16/18 |
| n = 68 | 91.2% | 93.5% | 96.8% | 94.4% | 93.8% | 92.9% | 92.1% | 88.9% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| 24 months | 57/65 | 26/29 | 25/30 | 11/17 | 26/29 | 14/15 | 26/28 | 12/15 |
| n = 65 | 87.7% | 89.7% | 82.3% | 64.7% | 89.7% | 93.3% | 92.9% | 80% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| 36 months | 48/58 | 20/23 | 23/24 | 14/23 | 27/30 | 9/10 | 25/26 | 6/7 |
| n = 58 | 82.7% | 86.9% | 95.8% | 60.9% | 90% | 90% | 96.2% | 85.7% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.004 | p < 0.001 | p < 0.04 | |
| 48 months | 42/50 | 21/23 | 18/20 | 8/17 | 13/19 | 6/6 | 22/23 | 5/5 |
| n = 50 | 84% | 91.3% | 90% | 47.1% | 68.4% | 100% | 95/6% | 100% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 | |||||
| 60 months | 49/62 | 23/28 | 22/24 | 11/19 | 19/36 | 6/6 | 28/32 | 5/5 |
| 79% | 82% | 91.7% | 58% | 52.8% | 100% | 87.5 | 100% | |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.003 | p < 0.001 | p < 0.04 | p < 0.001 | ns |
| Time after TFS . | Cure of prolapse . | Cure of SUI . | Cure of urgency . | Cure of nocturia . | Cure of day time frequency . | Cure of dragging pain . | Cure of dysuria . | Cure of fecal incontinence . |
|---|---|---|---|---|---|---|---|---|
| 12 months | 62/68 | 29/31 | 30/31 | 17/18 | 30/32 | 13/14 | 35/38 | 16/18 |
| n = 68 | 91.2% | 93.5% | 96.8% | 94.4% | 93.8% | 92.9% | 92.1% | 88.9% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| 24 months | 57/65 | 26/29 | 25/30 | 11/17 | 26/29 | 14/15 | 26/28 | 12/15 |
| n = 65 | 87.7% | 89.7% | 82.3% | 64.7% | 89.7% | 93.3% | 92.9% | 80% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| 36 months | 48/58 | 20/23 | 23/24 | 14/23 | 27/30 | 9/10 | 25/26 | 6/7 |
| n = 58 | 82.7% | 86.9% | 95.8% | 60.9% | 90% | 90% | 96.2% | 85.7% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.004 | p < 0.001 | p < 0.04 | |
| 48 months | 42/50 | 21/23 | 18/20 | 8/17 | 13/19 | 6/6 | 22/23 | 5/5 |
| n = 50 | 84% | 91.3% | 90% | 47.1% | 68.4% | 100% | 95/6% | 100% |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 | |||||
| 60 months | 49/62 | 23/28 | 22/24 | 11/19 | 19/36 | 6/6 | 28/32 | 5/5 |
| 79% | 82% | 91.7% | 58% | 52.8% | 100% | 87.5 | 100% | |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.003 | p < 0.001 | p < 0.04 | p < 0.001 | ns |
These data included the learning curve. Observed cure rate is given in %.
McNemar’s test chi-square with one degree of freedom.
ns, >0.05.
The Pictorial Algorithm
Accurate diagnosis of ligament damage is fundamental to treatment. The pictorial algorithm (as shown in Fig. 2) relates specific symptoms to organ prolapse and causative suspensory ligaments. The predictable grouping of symptoms in the algorithm is a reliable guide to ligament causation of prolapse and symptoms, especially with minimal prolapse. It can be used directly to diagnose ligamentous cause of symptoms which can then be tested by “simulated operations,” applying mechanical support via the vagina to observing the change in symptoms (as shown in Fig. 5, 6).
“Simulated Operations”– Making a More Certain Diagnosis prior to Surgery
Surgery for Symptoms and Organ Prolapse
Simple surgical principles underlie the ITP system of surgery: conservation of uterus and vaginal tissue and repair of weak or loose ligaments with collagen-creating methods. These can be performed by native tissue or collagen-creating techniques. Some of the extensive data which supports the role of the 5 main damaged ligaments PUL, USL, cardinal, ATFP, perineal body ligaments in the causation of prolapse and bladder/bowel/pain symptoms predicted by the algorithm (as shown in Fig. 2), is detailed in references [13‒50]. The question addressed in this section is, “Is native ligament repair sufficient for ligament repair?”
When to Perform Native Tissue or Collagen-Creating Surgical Techniques?
Comparison of Tables 1 and 2 goes some way to answering this question and supporting the ITP’s fundamental thesis of collagen deficiency in ligaments being at the core of prolapse and bladder/bowel/pain dysfunctions. The very poor results for native ligament repair of prolapse, urge, frequency, nocturia symptoms in postmenopausal women at 18 months (Table 2; Shkarupa et al. [38]) contrast starkly with the high 5-year cure rates for pelvic symptoms following sling repair by Inoue et al. [35] (Table 1). This comparison supports the inevitable conclusion reached by Shkarupa et al. [38], that for postmenopausal women, a collagen-creating method such as a sling is required to achieve longer-term results.
Shkarupa et al. [38] compared two groups who had native cardinal and USL repair, premenopausal (n = 49) and postmenopausal (n = 39)
| POP/OAB symptoms . | Premenopausal group (n = 49) . | Postmenopausal group (n = 39) . |
|---|---|---|
| 3 months | ||
| Frequency | 71,5 | 64.1 |
| Urgency | 85.7 | 82 |
| Nocturia | 96 | 64.1 |
| POP | 98 | 89.7 |
| 6 months | ||
| Frequency | 77.5 | 48.7 |
| Urgency | 85.7 | 64.1 |
| Nocturia | 98 | 59 |
| POP | 85.7 | 48.7 |
| 12 months | ||
| Frequency | 63.3 | 38.5 |
| Urgency | 81.6 | 33.3 |
| Nocturia | 71.5 | 25.6 |
| POP | 85.7 | 20.5 |
| 18 months | ||
| Frequency | 59.2 | 15.4 |
| Urgency | 67.3 | 17.9 |
| Nocturia | 87.7 | 20.5 |
| POP | 79.6 | 15.4 |
| POP/OAB symptoms . | Premenopausal group (n = 49) . | Postmenopausal group (n = 39) . |
|---|---|---|
| 3 months | ||
| Frequency | 71,5 | 64.1 |
| Urgency | 85.7 | 82 |
| Nocturia | 96 | 64.1 |
| POP | 98 | 89.7 |
| 6 months | ||
| Frequency | 77.5 | 48.7 |
| Urgency | 85.7 | 64.1 |
| Nocturia | 98 | 59 |
| POP | 85.7 | 48.7 |
| 12 months | ||
| Frequency | 63.3 | 38.5 |
| Urgency | 81.6 | 33.3 |
| Nocturia | 71.5 | 25.6 |
| POP | 85.7 | 20.5 |
| 18 months | ||
| Frequency | 59.2 | 15.4 |
| Urgency | 67.3 | 17.9 |
| Nocturia | 87.7 | 20.5 |
| POP | 79.6 | 15.4 |
A Tape May Not Be the Only Way to Create New Collagen
Calculation from Instron testing data from a rejected aortic graft, part of the first author’s Doctor of Surgery Thesis on “The Development of the Midurethral Sling” (University of Western Australia, 1999) indicated that neocollagen production from No. 2 wide-bore polyester sutures would be sufficient to repair weakened PUL ligaments and cure SUI by two orders of magnitude [53]. Initial testing on discharge from hospital for a new urethral ligament plication operation, which plicated all 3 parts of PUL via parallel incisions in the distal sulci of the vagina, gave high cure rates for SUI [54]. Cure rate from objective testing at 12 months in a registered study (n = 35) was 83% [55]. The wide-bore polyester suture method has been successfully applied to cardinal/uterosacral Fothergill-type repairs and for repair of major perineoceles with descending perineal syndrome (as shown in Fig. 4).
Can Muscle Damage Cause Pelvic Symptoms?
Though the ITP emphasizes collagen as its weakest (and most correctible) link, it must be remembered that it is the pelvic muscles which act against the ligaments to activate all 3 aspects of pelvic floor function: urethral/anal closure, evacuation, prevention of premature evacuation (urge) (Figure 3) [1]. Ligament cure of symptoms is clearly not the whole answer for reported symptom cures [15‒50]. Individual cure rates, for example, urge and nocturia, range from 65% to 85% [15‒50]. A critical blinded experiment in 47 women having a midurethral sling showed very significant muscle damage in the majority of the 47 pelvic muscle specimens examined [14]. In that study, 33/39 SUI cases (85%) were cured of SUI the morning after their midurethral sling, indicating the key role of the PUL as a competent anchoring point for the pelvic closure muscles [14]. However, 6 women (15%) were not cured [14]. This experiment indicates a potential role for nerve and muscle damage in SUI pathogenesis. Studies by Swash et al. [56] demonstrated nerve damage in women with bowel and bladder dysfunctions, which improved with time after delivery.
Cure of Ongoing Massive Incontinence from Obstetric Fistula Scarring after Closure
Application of the ITP’s “tethered vagina syndrome” protocols solved a major problem in women with massive ongoing urine loss following successful obstetric fistula closure. With reference to Figure 3, it is evident that there has to be sufficient elasticity in the bladder neck area of the vagina, “zone of critical elasticity,” for the opposite muscle forces, pubococcygeus muscle anteriorly, and LP/LMA posteriorly, to act independently [1]. Scarring from an obstetric fistula in the anterior vaginal wall “tethers” the more powerful LP/LMA directly to the pubococcygeus muscle and forces it open, so up to 40% of women with successfully closed obstetric fistula leak urine constantly. A skin graft applied to the bladder neck area of the vagina prophylactically and therapeutically, restores vaginal elasticity [57]. Using the Singapore flap skin graft, Dr. Browning et al. [58, 59], a fistula surgeon, has produced dramatic restoration of continence in a large number of such women.
Non-Surgical Cure/Improvement of LUTS
The fundamental purpose of ligament repairs is to provide firm anchoring points for the 3 reflex forces which close and open the urethra and anus (as shown in Fig. 3). Figure 5 shows how mechanical support of PUL can temporarily abolish SUI, and Figure 6 similarly for urge and pelvic pain. Ring pessaries demonstrated pain relief in 50% of women tested [47].
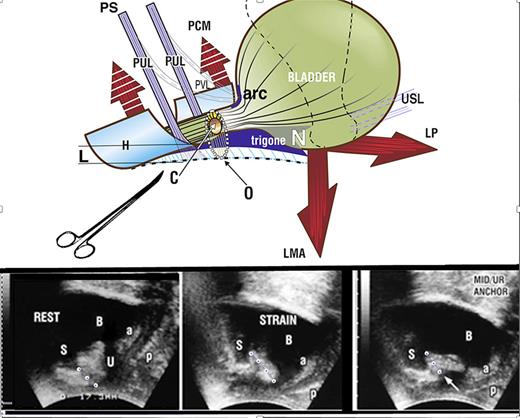
Restoration of continence by PUL support. Upper image shows SUI. If PUL is weak, it lengthens to “L” on effort and cannot hold the posterior urethral wall and vagina (to which it is attached) against the powerful LP/LMA opening forces; PCM cannot stretch the distal vaginal hammock “H” forward sufficiently to close the distal urethra from behind; LP/LMA forces stretch the trigone backward to open “H” and the posterior urethral wall, from “C” (closed) to “O” (open), broken lines. The hemostat mimics what a suburethral sling does; it prevents PUL extension to “L” and restores urethral closure, as seen in the right ultrasound frame (white arrow). Lower images show transperineal ultrasound in a patient with SUI. Rest (left image): three small white circles represent PUL. The organs are in the correct anatomical position. S, symphysis; U, urethra; a & p, anterior and posterior vaginal walls. Strain (middle image): note elongation of PUL on straining (4 white circles). The funneling of the bladder neck and expansion of the urethral diameter along the whole urethra exponentially lowers the urethral resistance to urine flow and urine is lost on coughing. Hemostat test (right image): the white arrow represents the hemostat. The hemostat mechanically supports a weak PUL, prevents elongation “L”, and prevents opening by LP/LMA to cause SUI.
Restoration of continence by PUL support. Upper image shows SUI. If PUL is weak, it lengthens to “L” on effort and cannot hold the posterior urethral wall and vagina (to which it is attached) against the powerful LP/LMA opening forces; PCM cannot stretch the distal vaginal hammock “H” forward sufficiently to close the distal urethra from behind; LP/LMA forces stretch the trigone backward to open “H” and the posterior urethral wall, from “C” (closed) to “O” (open), broken lines. The hemostat mimics what a suburethral sling does; it prevents PUL extension to “L” and restores urethral closure, as seen in the right ultrasound frame (white arrow). Lower images show transperineal ultrasound in a patient with SUI. Rest (left image): three small white circles represent PUL. The organs are in the correct anatomical position. S, symphysis; U, urethra; a & p, anterior and posterior vaginal walls. Strain (middle image): note elongation of PUL on straining (4 white circles). The funneling of the bladder neck and expansion of the urethral diameter along the whole urethra exponentially lowers the urethral resistance to urine flow and urine is lost on coughing. Hemostat test (right image): the white arrow represents the hemostat. The hemostat mechanically supports a weak PUL, prevents elongation “L”, and prevents opening by LP/LMA to cause SUI.
Strengthening Pelvic Symptoms by Squatting-Based Exercises
The squatting-based Skilling pelvic exercises aim to strengthen the 3 reflex pelvic muscles and the PUL and USL ligaments against which they contract (as shown in Fig. 3) [60]. These exercises gave an objectively tested improvement rate of more than 50% for SUI, urge, nocturia, frequency, CPP in mainly premenopausal women. Modified Skilling protocols were applied as an RCT in a group of 48 children aged 6–11 years with day/night enuresis. A cure rate of 86% was recorded for both conditions, plus major improvement in constipation [61]. Validation of this high cure rate by Pillai and Sara [62], indicates the role of the ITP in another (unexpected) revolution, cure of day/night enuresis in children [61].
Is the ITP Compatible with ICS Definitions and Methods?
Though the ITP has developed into a parallel paradigm to existing thought, it has, in fact, followed ICS definitions and has used standard ICS urodynamics extensively in all its discoveries. Two important examples of usage of standard ICS urodynamics were in 1993 [63] and in 1999 [64]. Standard cystometric and urethral pressure studies challenged by a handwashing test to block cortical control demonstrated that the sequence of events for DO (urodynamic detrusor overactivity) were identical to what happens during a normal micturition [63]. A similar test applied to women who had low compliance led to the discovery of a chaotically controlled binary bladder feedback control system (as shown in Fig. 3) [64]. The low compliance was found to be a prematurely activated, but controlled micturition reflex [65].
A major anatomical analysis to check whether the ITP as it exists today was performed in 2022 to check its compatibility with current ICS definitions [65]. Other than a minor change of OAB from “overactive” to “overactivated,” the anatomical explanations offered by the ITP were able to explain all the ICS definitions with full logical consistency [65]. The reason for recommendation to change to “overactivated” was it moves the focus of causation from the bladder itself to causes outside of the bladder [1, 65].
Conclusions
The ITP is a matrix which knits together the different strands of the female pelvic floor: brain, nerves, muscles, ligaments, in a holistic, cortically co-ordinated way (as shown in Fig. 3). The ITP as it is today (as shown in Fig. 1), is the work of many physicians over a period of 30 years. Many of the discoveries came from observing the fate of pelvic symptoms following ligament repair. Other discoveries such as cure of CPP, vulvodynia, OAB, DO, interstitial cystitis/bladder pain syndrome, fecal incontinence, obstructive defecation, anterior rectal wall intussuception, invalidation of the pressure transmission theory, squatting-based pelvic floor exercises, cure of day/night enuresis, cure of ongoing urine leakage after obstetric fistula closure came from experiments which directly challenged the ITP’s predictions.
Since its inception in 1990, as a theory, the ligament repair system has grown into a self-contained, entirely anatomical parallel universe encompassing the full range of bladder/bowel/pain/prolapse function, dysfunction and treatment. Collagen is at the core of this system: “Repair the structure and you will restore the function.” Importantly, it uses, and is compatible with, ICS definitions and provides anatomical explanations for each ICS definition.
An effective start for surgical cure/improvement of pelvic symptoms in women with pelvic organ prolapse (as shown in Fig. 2), can be made immediately by interested surgeons, using standard operations such as midurethral slings and modified tissue-conserving Manchester operations (as shown in video, modified Manchester operation: https://youtu.be/pEa61sWHkaQ?si=U-s-hXYYZP__oDyn).
Acknowledgments
The number of authors who have contributed to the body of knowledge of the Integral Theory Paradigm in the 35 years of its existence, either by original discoveries, or by validating bladder/bowel/pain/prolapse symptom cure by ligament repair, is far too extensive to acknowledge individually. It suffices to quote Thomas Kuhn [12] that “new paradigms are rarely, if ever the product of one person” and to thank each one of many hundreds globally.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
P.P., B.L., A.A.S., K.G., H.I., Y.S., B.A., D.S., and N.K. contributed to investigations leading to conceptualization and substance of the article. P.P. and B.L. wrote the original draft. A.A.S., K.G., H.I., Y.S., B.A., D.S., and N.K. contributed to checking, reviewing, additions, and editing. P.P. contributed to figures and resources.
Data Availability Statement
Additional resources include bladder/bowel/pain etiopathogenesis and minimally invasive treatment: 2024 Integral Theory Update (https://atm.amegroups.org/issue/view/1400); 3rd edition textbook, The Female Pelvic Floor, Function, dysfunction and Management, according to the Integral Theory (https://www.ics.org/education/icspublications/library); The 1990 Integral Theory of Female Urinary Incontinence (Petros & Ulmsten) and the experiments which support it, detailed at https://obgyn.onlinelibrary.wiley.com/toc/16000412/1990/69/S153; The 2008 Anorectal Theory (Petros & Swash), and the experiments which support it, detailed at https://www.researchgate.net/publication/267778578_The_MusculoElastic_Theory_of_anorectal_function_and_dysfunction; and chronic pelvic pain issue (https://cms.galenos.com.tr/Uploads/Article_39889/Pelviperineology-36-66-En.pdf).