Abstract
Introduction: Female stress urinary incontinence (SUI) is often treated surgically. Urethral bulking agents are a minimally invasive alternative, especially in patients suffering from intrinsic sphincter deficiency, but often with limited long-term efficacy. Urolastic® is a non-deformable, non-resorbable silicone elastomer that is used as an injectable. Its properties might result in a more durable response after injection. If this durability factor can be combined with a low complication rate, this can become a useful treatment option. We therefore assessed the subjective improvement and safety after treatment with Urolastic®. Materials and Methods: In 2 Dutch hospitals, 65 patients were treated with Urolastic®. The subjective improvement was assessed and the medical charts were reviewed for complications that appeared during the follow-up period. The complications were classified using the Clavien-Dindo classification. Results: We found that 76-88% of the patients showed subjective improvement at 12-25 months follow-up. The rate of improvement experienced was 50-70%. The rate of complications classified as Clavien-Dindo >II was 24-33%. The 12 patients with 75-100% subjective improvement after 2 months, showed 85% improvement after a median of 25 months. Conclusions: With careful patient selection, Urolastic® seems to be a safe, durable and effective treatment option for female SUI.
Introduction
Female stress urinary incontinence (SUI) is a common medical condition with a significant impact on the quality of life (QoL) [1]. According to most guidelines, after conservative measures, a midurethral sling (MUS) procedure is the treatment method of first choice [2]. For patients in whom sling surgery failed or for those who are unfit for surgery, options are limited. Urethral bulking agents (UBA) offer an alternative treatment option, albeit with variable clinical success rates [3]. Many of these products have been developed in the past decades; most of them have had limited efficacy, or have had problems with migrating particles or have resulted in other severe complications [4]. An ideal bulking agent should be durable, biocompatible, hypoallergenic, deformable, non-immunogenic, with minimal inflammatory response and should cause no migration [4,5].
Urolastic® (Urogyn BV, The Netherlands) has been found to have some of the properties mentioned [6,7]. This silicone elastomer (crosslinked vinyl dimethyl polydimethylsiloxane, VDPDMS) changes within minutes to a solid implant once injected into the paraurethral tissue. It has been used for years in hysteroscopical sterilization - by blocking the fallopian tubes - and shows good biocompatibility. No allergic reactions have been described and the inflammatory response is minimal. Because there are no microparticles, the risk of migration to other tissues is negligible.
If the properties mentioned above are combined with durable clinical results and low complication rates, Urolastic® might present a useful extra treatment option for female SUI. The objective of this paper is therefore to describe the extent and duration of the effect of Urolastic® and to evaluate the numbers and types of complications encountered after treatment with this implant.
Methods
In this retrospective cohort study, 65 patients were included. They were consecutively treated with VDPDMS in 2 Dutch hospitals: the “Groene Hart Ziekenhuis,” a general hospital (GH) in Gouda, and the Radboudumc, a tertiary referral centre (TRC) in Nijmegen. These centres were chosen because they treated relatively high volumes of patients and because it made comparison between different groups of patients - that is, primary- versus secondary-treated patients - possible. Patients were considered primary if they had not received any previous surgical treatment for SUI and/or prolapse. Treatments for urge incontinence or other pelvic surgery like hysterectomy were documented but not documented for the decision-making of a subject as “primary” or “secondary” patient.
In all patients, medical history was gathered and patients underwent physical examination to confirm the presence of SUI. Only in the TRC, urodynamics were usually performed preoperatively. After SUI was observed and diagnosed, the patients received 4 para-urethral injections (at 2, 5, 7 and 10 o'clock) at the level of the midurethral. The volume of material injected was 0.6-1.2 mL per position. Theoretically, the depositions at the 5 and 7 o'clock positions were always 0.2 mL larger than the depositions at 2 and 10 o'clock positions to give more support to the urethra. If urodynamics were available, the injected volume was sometimes adapted to the urinary flow measured preoperatively and the contractility of the bladder. Urologists with an experience of at least 5 procedures performed the procedures, mostly under local anesthesia during an outpatient procedure. At the end of the procedure a cough test confirmed the absence or reduction in the intensity of SUI.
A follow-up visit was scheduled at 6-8 weeks after injection. If the outcome of the first session was not satisfactory, additional injections were optional. Follow-up started from the day of the last procedure.
In February and March 2016, the subjective improvement was assessed through a telephone survey. All patients with the implants still in place were asked for the perceived percentage of improvement. In addition, we asked for the Patient Global Impression of Improvement (PGI-I). This is a transition scale that ranges from 1 (very much better) to 7 (very much worse). This scale has been tested for use in SUI and has a good degree of construct validity [8].
At the same moment, we retrospectively analyzed the charts for complications that had occurred due to the procedure. Complications were subsequently rated using the Clavien-Dindo classification of surgical complications, which is proven to be valid, is easy to use, gives reproducible results [9] and is widely used in the field of urology [10]. In this scale, the complications are rated from grade I to V, with grade I being “any deviation from the normal postoperative course” and grade V being “death of a patient.”
Patients
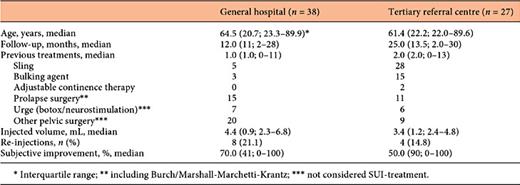
In the GH group, 38 women were included (Table 1), 29 of whom were primary patients, that is, they did not receive any treatment for SUI and/or prolapse.
Of the 9 non-primary patients, 5 patients had had a sling procedure with tension free vaginal tape (TVT)-obturator (TVT-O) or TOT, 2 had been treated with other bulking agents (Contigen® and Macroplastique®) and 2 had undergone only surgical prolapse repair. In total, 15 additional surgical procedures had been performed for prolapse and/or SUI: sacrospinous fixation (n = 3), anterior/posterior vaginal repair (n = 10) and Burch colposuspension/Marshall-Marchetti-Krantz (n = 2).
In the total GH-group, 4 patients had received botulinum toxine-injections for urgency or urge-incontinence and one had been treated with neuromodulation (PTNS). Other earlier urogynaecological procedures in this group of patients were hysterectomy (n = 10) and transurethral resection of a bladdertumor (n = 2). One patient had had radiotherapy.
Three women could not be reached by phone; one woman had the Urolastic® removed because of permanent retention. In the remaining 34 patients, a median follow-up of 12 months was reached.
In the group from the TRC, 27 patients were included (Table 1). This group consisted of very-difficult-to-treat patients, with a total of 71 previous procedures for incontinence or prolapse. Most patients revealed a fixed urethra on physical examination. Five patients had had no previous treatment for SUI and/or prolapse and were considered primary.
In the 22 non-primary TRC-patients, 15 patients had had one or more sling procedures with TOT, TVT-O, minitapes or fasciaslings. Twelve patients received one or more bulk injections, mostly with Zuidex® or Macroplastique®. In 10 patients, a more elaborate surgery for prolapse or SUI was performed: sacrocolpopexy (n = 3), Burch colposuspension/Marshall-Marchetti-Krantz (n = 6), anterior or urethral plasty/mesh repair (n = 4) and colpocleisis (n = 1).
Other prior urogynaecological procedures in the TRC-group were hysterectomy (n = 3), cystectomy (n = 2) and a Mitrofanoff stoma. For urge incontinence, patients were treated with botulinum toxine-injections (n = 5) and one had had a neuromodulation procedure. Two patients had undergone radiotherapy prior to receiving the injection.
In 5 out of 27 patients, the material was removed during follow-up, thrice because of pain or erosion and twice in order to make other procedures possible after a failed attempt to treat SUI. One patient died due to an unrelated malignancy. In the remaining 21 patients, a median follow-up of 25 months was reached. The results are listed in Table 1.
Results
Subjective Improvement
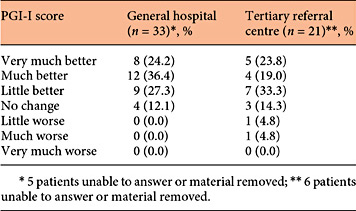
The subjective improvement was measured by a telephone survey asking for the perceived percentage of improvement and by scoring the PGI-I. In the GH, a median subjective improvement of 70.0% was found after a follow-up of 12.0 months (Table 1). Improvement of incontinence was reported in 29 out of 34 interviewed patients (85.3%). No patient reported a worsening of their symptoms (Table 2).
In the TRC, the subjective improvement of SUI was 50.0% after a median follow-up of 25 months (right column of Table 1). In this cohort, 16 out of 21 patients (76.2%) reported an improvement in symptoms of SUI (Table 2). There were 2 patients who reported a worsening of symptoms (9.5%).
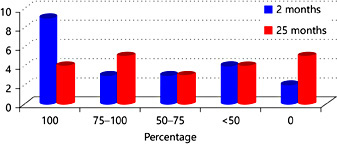
For the patients from the TRC, 6-8 weeks after treatment an improvement was observed which was also documented and these results are depicted in Figure 1. A sub analysis of the 12 patients who reported an improvement of 75-100% after 2 months showed a median subjective improvement of 85% after 25.5 months (Fig. 1). We found no other parameters that had a significant correlation with the outcome measures.
Subjective improvement in TRC after 2 versus median 25 months.
Subjective improvement in TRC after 2 versus median 25 months.
Complications
During the procedures, it is possible that the material gets injected in an unintended place. In both the GH and the TRC, one deposit ended up in the bladder. However, the material could in both cases be removed cystoscopically after the procedure. It was found that injection through the vaginal wall was also mistakenly done once in both centres. The deposit was removed immediately in these cases and during a follow-up visit, a reinjection was done at the respective positions.
Another complication encountered directly after the procedure was de novo retention. In the GH, 6 patients (15.8%) had a retention that was treated with an indwelling catheter of 12G for 1-7 days. In one case, the retention was permanent and the material had to be removed in order to restore normal voiding. In the TRC, 3 patients (11.1%) had a retention. In 2 patients, this was resolved with an indwelling catheter for 3 days; 1 patient used clean intermittent catheterization (CIC) for 3 weeks after which she was able to void spontaneously again. Of the 27 patients treated in the TRC, 4 were regularly performing CIC before the procedure and continued to do so afterwards.
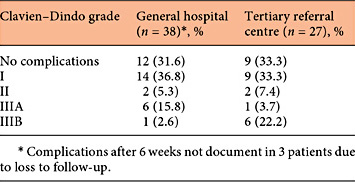
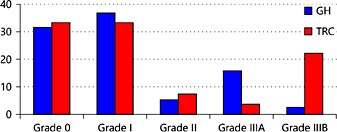
The complications found during follow-up were scored using the Clavien-Dindo classification (Table 3; Fig. 2). Three patients of the GH could not be reached by phone; thus, complications that might have occurred after 6-8 weeks were not documented in these 3 women. In the GH and TRC, 12 patients (31.6%) and 9 patients (33.3%) were complication-free respectively. A score of I was given to 12 patients (31.6%) in the GH and to 9 patients (33.3%) in the tertiary centre. Reasons were exposure or erosion of the implant, minor pain complaints, anti-emetics use or an overnight stay because of de novo retention. A further 2 patients in each centre had a score of II because of more severe complaints of pain or urgency. These complaints were treated pharmacologically and resolved afterwards.
In both centres, a total of 17 (24.6%) exposures or erosions were reported. An exposure was considered a part of the implant that was not covered by tissue. Some of them were a trail of material that had solidified in the injection channel. The trails can often be removed with a forceps without the need for anesthesia. Erosions are a delayed complication; these arise when implants erode through the tissue. This was mainly observed at the anterior vaginal wall and was left untreated in case there were no complaints. Transurethral erosion was seen in 2 patients: one had previously undergone radiotherapy and had a complicated procedure due to a frozen urethra; one had a history of multiple TVT procedures and performed CIC. The urethral erosions were removed cystoscopically.
The most serious complication in both centres was erosion of the material through the anterior vaginal wall and postoperative pain. In 7 out of 38 patients (18%) and 7 out of 27 patients (26%), this led to (partial) removal of the material under local or general anesthesia, which in turn gives a Clavien score of IIIA or B. Removal of the implants was done by a vaginal incision. In 2 women, removal of one of the implants at the 5 o'clock position did not lead to the recurrence of incontinence.
No significant correlations were found between the volume of bulk, the number of previous procedures or age and the complications observed.
Primary versus Secondary Patients
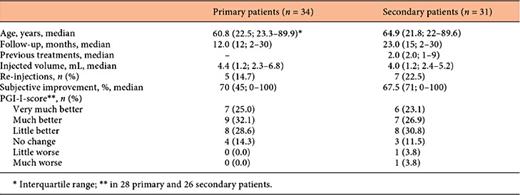
A sub analysis was made to compare the primary with the secondary patients from both centres. The results of this comparison are depicted in Table 4. No significant differences in characteristics and outcomes were found. The subjective improvement tends to be better in the primary patients.
Discussion
Bulking agents have a long and disputed history in the treatment of female SUI. Since the start of this type of treatment in 1938 [11], many materials and devices have been developed for this use. Regrettably most methods have limited efficacy, short duration of the effect or both. Moreover, complications are involved. The limited successes of these products led to a negative general image of urethral bulking agents among urologists and gynaecologists. Development of the MUS like the TVT, with long-term subjective success rates of around 80% [12], also contributed to a physician's preference for slings as a primary treatment for SUI.
SUI has 2 main contributing factors. One is intrinsic sphincter deficiency (ISD) and the other is urethral hypermobility [13]. When urethral hypermobility is present with or without ISD, a MUS is efficacious. However, when ISD is present without urethral hypermobility, tapes do not work that well. Other treatment options like urethral bulking procedures are then indicated. Classically, bulk is chosen in case of isolated ISD, based on the idea that bulk increases the urethral resistance and leads to the coaptation of the urethral lumen [14]. Several studies have shown, however, that not only in case of ISD but also in case of urethral hypermobility, patients can benefit from UBA procedures [15,16,17]. A meta-analysis by Leone Roberti Maggiore et al. [18] showed that although the objective cure after MUS procedures is better, the subjective outcomes are not significantly different. Women are willing to sacrifice some efficacy in order to get a less invasive procedure [19]. Although good results can be achieved with a second sling procedure after failure of the first [20], some patients do not wish to have this done or are simply not fit for (re-)surgery. For these patients, bulking agents might be the last resort of treatment.
Given the above, new injectables are being developed continuously. The withdrawal of the widely used Contigen® (bovine collagen) after allergic reactions was an extra motivation for the development of new agents. A new bulking agent should ideally possess certain specific properties [3,21]. First, the procedure should be safe and easy to perform. Second, the bulking agent itself has to be non-immunogenic, non-inflammatory and biocompatible. If it consists of microparticles, these should be larger than 80 µm so as to avoid migration to other tissues [22]. Finally, the effect should be long lasting, which in in practical terms means that it should be non-biodegradable. The materials that are now being used in clinical practice do not entirely fulfill the criteria for an ideal bulking agent. The main reason for this is the low durability of the effect of treatment. This leads to repeated injections and relatively high costs.
Besides Urolastic®, there are presently 4 other bulking agents that have CE and/or FDA approval: Bulkamid®, Coaptite®, Durasphere®, and Macroplastique®. Comparison with these products is difficult due to different outcome measures used in the respective studies. Furthermore, the scarce comparative studies published use Contigen® as a reference, which is no longer available. Since collagen is no longer available, we chose to compare the outcome of our study with the most widely used bulking agents in Europe: Macroplastique® and Bulkamid®.
In this study, we found that 88% of the patients in the GH showed subjective improvement at a median of 12 months follow-up. The rate of complications classified as Clavien-Dindo >2 was 24%. Among the patients treated in the TRC, with many patients that had had more surgical procedures for SUI and in whom the application of bulk was a last resort treatment, the improvement rate was 76% after a follow-up of median of 25 months. The complication rate was 33% in this centre.
Ghoniem et al. [23] published a systematic review and meta-analysis of Macroplastique® in 2013. This review included 958 patients from 24 articles and reported on the short-, mid-, and long-term cure and improvement, as well as reinjection and complication rates. A mid-term (6-18 months) improvement rate of 73% was found, compared to an 88% improvement rate of Urolastic® in the GH at 12 months follow-up. A long-term (>18 months) improvement rate for Macroplastique® of 64% was reported, in our study; 76% of the patients in the TRC reported an improvement after median 25 months follow-up. The reinjection rate described by Ghoniem was 30%; in our study, this rate was on average 18%. We found a rate of serious complications (Clavien-Dindo >2) of 18% in the GH group and 26% in the TRC, mainly due to the removal of (a part of) the implants under local or general anesthesia. In the meta-analysis of Ghoniem [23], no serious adverse events were recorded.
In a systematic review performed by Kasi et al. [24] in 2016, 8 studies were included with a total of 767 patients who were treated with Bulkamid®. The average reinjection rate was 24% compared to 18% in those treated with Urolastic®. The subjective improvement in QoL questionnaires in the included studies of this review, showed an improvement of average 68% (range 40-100%) after one year follow-up. This is comparable to our results in the GH, where a median subjective improvement was found to be 70%. No serious treatment-related complications were identified in the included articles. The total number of adverse events, if given, ranged from 23 to 56%. This was 67-68% in our study.
Due to the non-availability of objective outcome measures for Urolastic® in our study, comparison of these measures with the other bulking agents is not possible. Another shortcoming of this study is its retrospectivity, which forced us to use the PGI-I and the subjective percentage of improvement, as no baseline data with validated QoL questionnaires were available. This study does, however, give a good indication of what the effects on incontinence can be of this intervention in 2 different groups of patients after a longer period of time.
The results seem to show that Urolastic has a relatively high complication rate. We found rates of 24% in the GH and 33% in the tertiary centre (i.e., a Clavien-Dindo score of II or higher and that required a pharmacological or surgical intervention). The most serious complication that was observed in our study was the exposure of the implants through the anterior vaginal wall, which led to a IIIA or IIIB classification in case of removal under local or general anesthesia. Though serious, this procedure is in most cases easy to perform, as the tissue shows no ingrowth in the implanted material and a small vaginal incision is sufficient to remove the entire implant.
The loss or displacement of one of the implants - either through surgery or spontaneously - did not necessarily cause renewed SUI. In one patient, an implant was displaced proximally during surgery; however, she retained full continence. This finding raises questions about the mechanism of action of Urolastic®. Urethral support possibly plays a role in regaining continence as well as urethral coaptation. However, the number of patients treated is small and more urodynamic research with urethral pressure profiles is necessary to assess the effect of Urolastic® on maximal urethral closing pressure (MUCP). Women with a lower MUCP have a higher chance of experiencing SUI [25] and it would be interesting to see what influence Urolastic® has on this parameter.
In 2 other studies published on Urolastic® similar complication rates were observed. A complication rate of 30% was reported by Zajda and Farag [6] in a similar group of 24 patients. In their study, there was a follow-up of one year. In contrast to our study, no mention is made of exposures. The most serious complications were hematoma, retention and vaginal pain. Vaginal pain was partly caused due to the injection of bulk at the 6 o'clock position. It seems that injecting at the 5 and 7 o'clock positions instead of the 6 o'clock position diminishes this risk. Implants that caused pain were removed with an effortless procedure, using a small vaginal incision. The second study published is from Futyma et al. [7], who treated a group of 105 patients, 91 of whom were recurrent patients. A complication rate of 16.2% was reported and no serious adverse events were mentioned. The implants were removed without effort because of pain or retention in 10 patients. In one patient, Urolastic® was removed from the bladder, but no vaginal wall erosions were seen after a follow-up of one year.
Despite the complications that do occur, implantation of Urolastic seems to be a safe procedure, without migration or unwanted tissue reactions. This is combined with an overall subjective improvement of 50-70% depending on the types of patients. In the group of patients with an initial success after 6-8 weeks, the improvement seems to be durable, with an 85% median improvement after more than 25 months of follow-up. This suggests that if we can select the right patients, we might be able to get higher cure and improvement rates. A larger prospective study with subjective and objective outcome measures and a longer follow-up duration is necessary to confirm the durability of the effect and to identify the patients most eligible for treatment with this procedure.
Conclusion
Urolastic has a subjective improvement of 50-70% depending on the types of patients treated. This effect is furthermore durable, with patients being continent after >2 years of follow-up.
Complications do occur. Most complications are resolvable but exposures remain a serious challenge in this procedure.
With careful patient selection, Urolastic® seems to be a safe, durable and effective treatment for female SUI.
Disclosure Statement
This study was supported by an unrestricted grant from Urogyn BV, Nijmegen, The Netherlands.