Abstract
Introduction: The aim of this study was to prove if the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic resulted in a delay in diagnosis and treatment of prostate cancer (PC). Methods: A monocentric, retrospective analysis was conducted at a university cancer center. Included were all patients with untreated PC diagnosed between January 2019 and December 2021. The observation covered 22 months of the SARS-CoV-2 pandemic and 14 months preceding it. Results: Nine hundred sixty-nine men prior (T0) and 1,343 during the pandemic (T1) were included. Mean age was 68.0 (SD 8.2). Median initial prostate-specific antigen was 8.1 ng/mL (T0) and 7.9 ng/mL (T1, p = 0.288). Time from biopsy to tumor board (T0: 1.3 months vs. T1: 0.9 months, p = 0.001), to staging (T0: 1.1 months vs. T1: 0.75 months, p = 0.707), and to therapy (T0: 3.0 months vs. T1: 2.0 months, p < 0.001) was shortened during the pandemic. Classified by d’Amico, a significant shift toward higher risk groups was seen (p = 0.024). Local staging showed an insignificant increase in locally advanced PCs. Metastatic diseases decreased from 10.3% to 8.9% (p = 0.433). Pathological staging showed pT3+ in 44.4% versus 44.7% (p = 0.565) and pN+ in 9.9% versus 9.6% (p = 0.899). Conclusion: Regarding the diagnosis and treatment of PC, we could not demonstrate any delays due to the SARS-CoV-2 pandemic.
Introduction
In January 2020, the world was confronted with the emergence of a new and highly contagious virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the coronavirus disease (COVID-19). The World Health Organization (WHO) confirmed the presence of this novel virus in Wuhan, China [1], and on January 28, 2020, the first case was reported in Germany [2]. This marked the beginning of a global health crisis of unprecedented proportions. As the weeks passed, SARS-CoV-2 quickly spread throughout Germany and beyond, causing a surge in COVID-19 cases worldwide [1]. Faced with this growing threat, the German government took swift action to curb the spread of the virus, implementing stringent contact restrictions, social distancing measures, travel limitations, and quarantine protocols in March 2020 [3]. Furthermore, medical facilities were urged to prioritize urgent cases, and elective therapies were postponed to alleviate the strain on healthcare resources [3, 4]. The impact of the pandemic on the healthcare system was profound. Hospitals became overwhelmed with COVID-19 patients, leading to delays and disruptions in non-urgent medical treatments and routine screenings [5]. Many patients faced challenges in accessing timely diagnosis and treatment for non-COVID-19 health conditions, as hospitals grappled with limited capacities, staff shortages, and patient concerns about visiting healthcare facilities [5]. The initial lockdown measures appeared to be effective, and in May 2020, Germany cautiously eased restrictions, as the spread of SARS-CoV-2 seemed to be under control. Medical treatment for elective indications was no longer restricted, providing some relief to patients in need of non-urgent care. However, the respite was short lived, as a resurgence of COVID-19 cases prompted the gradual reintroduction and intensification of contact restrictions from October 2020 to February 2021 [4, 6, 7]. Once again, surgical capacity was curtailed, particularly in regions with high incidence rates of SARS-CoV-2, putting further strain on the healthcare system [4, 6, 7]. Amidst the uncertainties surrounding surgical capacity and postponed elective surgeries, one critical area of concern remained cancer screening. Despite the challenges posed by the pandemic, cancer screening was not supposed to be compromised at any point by the government. However, European countries, including Germany, experienced negative impacts on cancer screening rates [7, 8]. The European Association of Urology (EAU) advised against routine prostate cancer (PC) screening, which involves prostate-specific antigen (PSA) testing and digital rectal examination, for asymptomatic individuals during the pandemic [4]. Reductions in cancer screening and a shift toward higher tumor stages upon detection were observed for various tumor types in different countries [9]. PC, in particular, presents a unique challenge as it typically grows asymptomatically, and its detection relies heavily on sequential check-ups involving physical examinations and PSA testing [10]. Given the disruptions caused by the SARS-CoV-2 pandemic on healthcare services, the objective of this study was to investigate whether delays in the diagnosis and treatment of PC occurred during this challenging period of time at a German University hospital.
Methods
A monocentric, retrospective cohort study of PC patients was carried out at the Prostate Cancer Center of the University Hospital of Regensburg, Germany. The study included all cases of untreated, newly diagnosed, histologically confirmed PC, that had been discussed in the multidisciplinary tumor board between January 2019 and December 2021. A chart-based follow-up was conducted for all patients to gather information on the date of prostate biopsy, tumor board discussion, staging imaging, treatment, and salvage treatment, if required. Additionally, the analysis incorporated the pathological staging and grading of both the prostate biopsy and prostatectomy specimens. These data were extracted from our datasets as thoroughly as possible. For patients who were only presented to our tumor board but received treatment elsewhere, the data were obtained by contacting the urologists who provided the treatment. Patients already in treatment prior to October 2019, including active surveillance (AS), were excluded from the study.
The observation period covered 22 months of the SARS-CoV-2 pandemic in Germany from March 2020 to December 2021 and the 14 months preceding it. The study period was divided into two segments: the period before the pandemic (T0: January 2019 to February 2020) and the period during the pandemic including the first lockdown, the interval between lockdowns and the second lockdown (T1: March 2020 to December 2021).
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) applying T test for continuous variables with normal distribution and Mann-Whitney U test for variables with non-normal distribution. χ2 test was used for binary variables. Continuous variables were reported as median and interquartile range (IQR) and categorical endpoints as absolute and relative frequencies. Significance was set at p values <0.05, and all analyses were two tailed.
Results
A total of 2,312 patients were screened. One hundred fifty-five patients were excluded, as these cases were follow-up visits within AS. A total of 2,312 men with untreated, histologically proven PC were included in the analysis, 969 prior (T0) and 1,343 during the pandemic (T1). Mean age was 68.0 (SD 8.2) years. Regarding the mean age, no difference (p = 0.181) was seen between the different observation periods; prior to the pandemic (T0), mean age at diagnosis of PC was 67.71 years (SD 8.22) and thereafter (T1) 68.22 years (SD 8.2). Median initial PSA was 8.1 ng/mL (T0, IQR: 7.87) and did not change significantly in the later period (T1) with a median PSA of 7.9 ng/mL (IQR: 7.71, p = 0.203). The median number of biopsy cores per patient increased during the observation period from 12.0 (IQR 3) in T0 to 14.0 (IQR 4) in T1 (p ≤ 0.001), corresponding to the significantly increased rate of ultrasound-guided MRI fusion biopsies, from 54.4% to 71.6% (p = 0.001). The proportion of tumor-infiltrated biopsy cores did not change significantly, T0: 33% (IQR 37%) versus T1: 33% (IQR 40%, p = 0.437).
Time to tumor board discussion (T0: mean 1.3 months vs. T1: mean 0.9 p ≤ 0.001) was not delayed, and it was even significantly shortened during the pandemic that applied equally to the median time from biopsy report to staging (T0: 1 month vs. T1: 1 month p = 0.665) and time to therapy (T0: 3.0 months vs. T1: 2.0 months, p ≤ 0.001). The median time interval between surgical treatment and, if necessary, adjuvant radiation was comparable before and during the pandemic (T0: 3.0 months; T1: 2.5 months; p = 0.576), as shown in Table 1.
Descriptive statistic
| . | Mean . | [SD] . | Median . | [IQR] . | Significance . |
|---|---|---|---|---|---|
| Age T0 | 67.71 | 8.213 | 0.181a | ||
| Age T1 | 68.17 | 8.244 | |||
| PSA level at initial diagnosis T0 (ng/mL) | 8.1 | 7.87 | 0.203b | ||
| PSA level at initial diagnosis T1 (ng/mL) | 7.8 | 7.71 | |||
| Number of taken biopsies T0, n | 12 | 3 | <0.001b | ||
| Number of taken biopsies T1, n | 14 | 4 | |||
| Number of affected biopsies T0 | 5 | 6 | 0.950b | ||
| Number of affected biopsies T1 | 5 | 6 | |||
| Percentage of affected biopsies T0 | 33% | 37% | 0.437b | ||
| Percentage of affected biopsies T1 | 33% | 40% | |||
| Distance between biopsy and tumor board T1 (months) | 1.38 | 2.73 | 1 | 1 | 0.002b |
| Distance between biopsy and tumor board T0 (months) | 0.61 | 0.79 | 1 | 1 | |
| Time interval between biopsy and first imaging T0 (months) | 1.05 | 2.86 | 1 | 1 | 0.707b |
| Time interval between biopsy and first imaging T1 (months) | 0.75 | 0.99 | 1 | 1 | |
| Time interval between biopsy and therapy T0 (months) | 3.00 | 2.10 | 3 | 1 | <0.001b |
| Time interval between biopsy and therapy T1 (months) | 2.42 | 1.41 | 2 | 1 | |
| Time interval between surgery and postoperative radiation T0 (months) | 3.04 | 1.34 | 3 | 2 | 0.576b |
| Time interval between surgery and postoperative radiation T1 (months) | 3.54 | 2.78 | 2.5 | 2 |
| . | Mean . | [SD] . | Median . | [IQR] . | Significance . |
|---|---|---|---|---|---|
| Age T0 | 67.71 | 8.213 | 0.181a | ||
| Age T1 | 68.17 | 8.244 | |||
| PSA level at initial diagnosis T0 (ng/mL) | 8.1 | 7.87 | 0.203b | ||
| PSA level at initial diagnosis T1 (ng/mL) | 7.8 | 7.71 | |||
| Number of taken biopsies T0, n | 12 | 3 | <0.001b | ||
| Number of taken biopsies T1, n | 14 | 4 | |||
| Number of affected biopsies T0 | 5 | 6 | 0.950b | ||
| Number of affected biopsies T1 | 5 | 6 | |||
| Percentage of affected biopsies T0 | 33% | 37% | 0.437b | ||
| Percentage of affected biopsies T1 | 33% | 40% | |||
| Distance between biopsy and tumor board T1 (months) | 1.38 | 2.73 | 1 | 1 | 0.002b |
| Distance between biopsy and tumor board T0 (months) | 0.61 | 0.79 | 1 | 1 | |
| Time interval between biopsy and first imaging T0 (months) | 1.05 | 2.86 | 1 | 1 | 0.707b |
| Time interval between biopsy and first imaging T1 (months) | 0.75 | 0.99 | 1 | 1 | |
| Time interval between biopsy and therapy T0 (months) | 3.00 | 2.10 | 3 | 1 | <0.001b |
| Time interval between biopsy and therapy T1 (months) | 2.42 | 1.41 | 2 | 1 | |
| Time interval between surgery and postoperative radiation T0 (months) | 3.04 | 1.34 | 3 | 2 | 0.576b |
| Time interval between surgery and postoperative radiation T1 (months) | 3.54 | 2.78 | 2.5 | 2 |
at test. bMann-Whitney U test.
T0, interval before the pandemic; T1, interval during the pandemic.
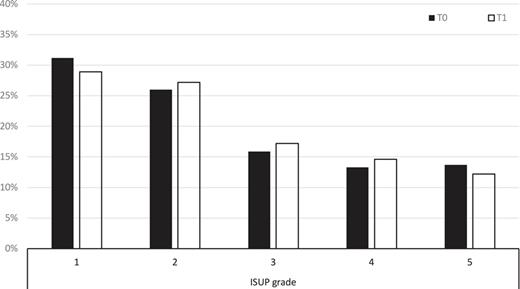
Prior to SARS-CoV-2 (T0), WHO grading was 1 in 31.2%, 2 in 26.0%, 3 in 15.9%, 4 in 13.3%, and 5 in 13.7%, respectively. This did not change significantly (p = 0.474) during the pandemic (T1) with WHO 1 in 28.9%, 2 in 27.2%, 3 in 17.2%, 4 in 14.6%, and 5 in 12.2%, respectively, as shown in Figure 1. A slight increase (71.1% T1 vs. 68.8% T0) in cases of significant carcinoma (WHO 2–5) did not demonstrate statistical significance (p = 0.25). Classified by d’Amico, a shift (79.4% T1 vs. 75.4% T0) toward higher risk groups (intermediate and high) was seen during the observation period (p = 0.024).
Changes in the ISUP grade during COVID-19. T0: interval before the pandemic; T1: interval during the pandemic. ISUP, International Society of Urologic Pathologists.
Changes in the ISUP grade during COVID-19. T0: interval before the pandemic; T1: interval during the pandemic. ISUP, International Society of Urologic Pathologists.
Staging
Data regarding a staging examination were available for 1,925 (83.4%) patients (T0: 797, T1: 1,127). In 15.8%, data for staging examinations were missing despite there being indicated. In T0, staging was conducted in 505 cases (52.5%). In T1, staging was conducted in 702 cases (52.5%). The number of patients receiving a staging examination did not significantly differ between the two observation periods (p = 0.705). The number of patients in whom a CT/MRI abdomen/pelvis had been conducted did not change over time and remained at approximately 60%. The same applies to skeletal scintigraphy with approximately 55%. However, the number of CT scans of the thorax significantly increased to 27.4% in the T1 period compared to 16.3% in T0 (p = 0.001). Approximately 3% of the patients receiving staging underwent a PSMA-PET-CT, and this percentage remained unchanged during both observation periods (p: 0.569).
In the local staging using CT/MRT/DRU, there was a slight increase in locally advanced prostate carcinomas (cT3), 2.1% (n = 17) at time point T0 to 2.8% (n = 33) at time point T1 (p: 0.501). Infiltration into neighboring organs (cT4) decreased from 1.4% (n = 14) to 1.1% (n = 11) over time. However, these changes were not statistically significant (p = 0.503).
Suspicious lymph nodes in pretherapeutic staging were observed in 11.3% (n = 32) at time point T0 and 9.8% (n = 67) at time point T1. However, this decrease did not show statistical significance (p = 0.387). The percentage of patients with metastatic diseases decreased insignificantly, from 10.3% (n = 52) with staging at time point T0 to 8.9% (n = 63) during the observation period T1 (p: 0.433). Upon closer examination, stable figures were observed for cM1a findings (2.0% at T0 vs. 2.4% at T1) and cM1b 5.1% (n = 26, T0) increased to 5.8% (n = 41, T1). Regarding cM1c findings, there was a slight decrease from 3.2% (n = 16, T0) to 0.7% (n = 5, T1).
Therapy
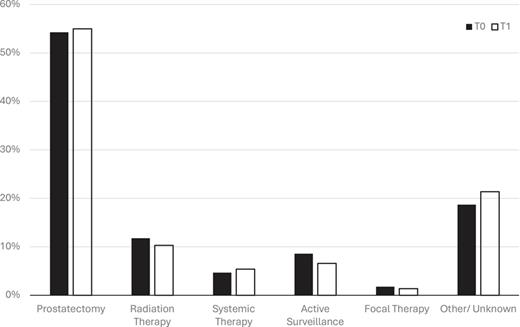
During the observation period T0, 298 patients (54.3%) opted for a radical robot-assisted prostatectomy (RARP), 65 patients (11.8%) chose radiation therapy, and 47 patients (8.6%) started AS. Ten patients (1.8%) received a focal therapy. The therapy for 103 patients (18.7%) remains unknown. Twenty-six patients (4.7%) started systemic therapy. In comparison, during the observation interval T1, 444 patients (55.0%) received radical prostatectomy, 83 (10.3%) chose radiation therapy, and 53 (6.6%) chose AS. Eleven patients (1.4%) received focal therapy, 44 (5.4%) started systemic therapy, and for 173 (21.4%), the therapy remains unknown (Fig. 2). There was no difference between the two observation periods (p = 0.604). The pathological results from prostatectomy specimen confirmed stable local extension with pT3+ in 44.4% (T0) versus 44.7% (T1, p = 0.565) and pN+ in 9.9% (T0) versus 9.6% (T1, p = 0.899), respectively. Positive surgical margins decreased from 36.3% (T0) to 29.6% (T1) during the pandemic (p = 0.06).
Changes in the frequency of therapies during COVID-19. T0: interval before the pandemic; T1: interval during the pandemic.
Changes in the frequency of therapies during COVID-19. T0: interval before the pandemic; T1: interval during the pandemic.
Discussion
This study aimed to evaluate whether the SarS-CoV-2 pandemic had an influence on the timing of PC diagnosis or therapy. We did not observe any time delay in terms of staging procedures or the initiation of therapy following diagnosis. There were also no significant shifts toward advanced or metastatic tumor conditions during the pandemic period compared to the period before the pandemic. The restrictions related to the COVID-19 pandemic indeed led to a significant decrease in the number of cases of SARS-CoV-2 infected patients; however, there was a notable reduction in the utilization of cancer screening examinations [11]. Worldwide, various restrictions on screening and therapy for PC were implemented during the COVID-19 pandemic. Vincentiis et al. [12] assessed the impact of the COVID-19 pandemic-related delay in the diagnosis of major cancers at a Secondary Care Hospital in Italy and were able to demonstrate a 75% decrease in newly diagnosed PCs. However, the low number of absolute cases (8 in the first 10 weeks during the pandemic compared to an average of 32 in the years 2018 and 2019 in the same period) and the short observational interval should be noted. Interestingly, Jacob et al. [11] who investigated the impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany were able to observe a significant overall decrease in cancer incidence of up to 40% in 1,600 general and specialized practices in Germany. However, for urological early detection, only a slight and statistically non-significant decline in initial diagnoses could be observed. In the New England area of the United States, during the first 3 months of the pandemic, a decrease in screening examinations and an increase in positive screening results compared to the same period in previous years were observed [5]. However, this could not be demonstrated in Australia. Here, in comparison, no significant changes in the numbers over the years (average from 2015 to 2019 compared to absolute numbers in 2020) for PSA tests, prostate MRI scans, and prostate biopsies were observed. However, they were able to show a significant decrease for the months with the most stringent lockdown phases (March to May 2020 and August 2020) [13]. In contrast to our findings, a survey conducted in urology practices in Brazil found a significant decline in the number of patient visits and surgeries during the pandemic [14]. We were unable to demonstrate a decrease in initial diagnoses in our cohort during the pandemic period. Additionally, we could not show that the SARS-CoV-2 pandemic led to a significant delay in the initiation of therapy in our cohort. On the contrary, we were able to demonstrate a statistically significant reduction in the time from biopsy to discussion in the tumor board and initiation of therapy. Possibly, this could be attributed to a quicker referral of patients to our center to keep patients out of the outpatient urological practice. In the observation period, a significant increase in the number of biopsy samples taken per patient could be observed. This is probably due to the significant increase in MRI-fused prostate biopsy procedures, which led to an overall rise in the number of biopsy samples taken (fused plus random). In Table 2, results from the most relevant studies conducted in various countries addressing the challenges in the care of prostate cancer patients during the pandemic are listed. A major issue in the analysis and assessment of the results is the chosen comparison intervals. Bakouny et al. [5] conducted a study describing the number of patients undergoing cancer screening tests and subsequent cancer diagnoses during the COVID-19 pandemic, defining three distinct comparison intervals: the first spanned 3 months before the beginning of the pandemic (from December 1, 2019, to March 2, 2020), the second covered the same 3-month period in the preceding year (from March 2 to June 2, 2019), and the third encompassed the 3 months following the main study period (from June 3 to September 3, 2020). Van Deukeren, who evaluated the impact of the COVID-19 outbreak on PC care in the Netherlands, compared the period from March to May 2020 with the same timeframe in 2019 (March to May). In contrast, Nguyen et al. [15] utilized a broader interval, comparing the period from March 23, 2020, to December 31, 2020, with the corresponding period from March 23, 2019, to December 31, 2019. We opted for a comparison interval of 14 months before the pandemic, against 22 months during the pandemic. This decision was made to have a correspondingly long reference period, but still close enough to the pandemic to avoid measuring general changes over the years. Additionally, the entire duration of the pandemic was combined to better capture potential changes over time. Because of the varying comparison intervals and the diverse and distinct restriction profiles implemented by each country during the pandemic, direct comparison of the results poses challenges. Another aspect to consider in PC studies is the natural progression of the disease, meaning that a delay in diagnosis or therapy does not necessarily lead to a deterioration in prognosis. Currently, there is no consensus on what constitutes an acceptable delay. The 2023 EAU guidelines provide a weak recommendation that the initiation of surgical therapy can be postponed by 3 months across all risk groups [15]. A review article about the clinical impact of delaying therapy for PC by Tachibana et al. [16] suggests delays ranging from 3 to 12 months as acceptable. Therefore, routine PC screening might be safely delayed for a period in the general population. The distribution of therapeutic modalities in our cohort regarding radical prostatectomy is in accordance with the literature. For instance, Herden et al. [17] in the HAROW study, a multicenter prospective observational study investigating the treatment of localized PC in everyday practice in Germany demonstrated that approximately 57% of patients underwent radical prostatectomy, 16% underwent radiation therapy, and 16% chose AS. We observed notably lower numbers for the active surveillance category. However, it is likely that a relevant number of patients with unknown therapy in our cohort fell into this group. As there was no further affiliation with an institute of our center, obtaining these data is challenging. We did not observe any change in the distribution of therapies during the pandemic. We did not observe a drop in radical prostatectomies during the pandemic. This might be related to two advantages of performing radical prostatectomy during the pandemic. On the one hand, the capacity for ontological surgery became available as elective non-cancer procedures were postponed. On the other hand, patients undergoing radical prostatectomy typically do not require intensive care, and there were no objections to performing the surgery due to intensive care unit capacity constraints. This was also demonstrated by Harke et al. [18] They conducted a multicentric survey-based study comparing the treatment of PC patients in the year 2020 with the year 2019 at urological and radiotherapy departments in Germany. Despite a decrease in treatments during the months with the strictest lockdowns, an overall increase in conducted treatments was observed throughout the year. The low rate of R1 findings in our cohort could be related to the higher rate of multiparametric MRI examinations and thus improved local staging. While most other research groups investigating the impact of COVID-19 on the diagnosis of PC utilized large national or international databases, resulting in higher patient numbers, our cohort’s strength lies in collecting our own data, allowing for a more in-depth and comprehensive evaluation. However, this study also has several limitations. Firstly, the chosen time interval should be mentioned. As discussed above, there is no consensus on what constitutes an adequate comparison interval. However, in the interval we selected, there is a risk that we are observing a random change compared to the year prior to the pandemic. To minimize the risk, we extended our observation period to 36 months, which to our knowledge is one of the longest study periods published. Another significant limitation is the monocentric design of this analysis. Due to the heterogeneous management of hospitals during the pandemic, the nationwide extent of shifts in diagnosis and therapy might not be fully represented.
The outcome of cancer patients in different COVID-19 studies regarding prostate cancer
| First author (year) . | Location . | Type of study . | Type of malignancy included . | Comparison period . | Patients included . | Main result . |
|---|---|---|---|---|---|---|
| Moschovas [19] (2021) | USA | Retrospective | PC, only patients with RP | March 1 to May 25, 2020, no comparison period | 147 | No patient with COVID-19 infection after RARP, 2 patients were rescheduled to positive COVID-19 test. RARP is safe and feasible during the pandemic |
| Oderda [20] (2021) | Italy | Retrospective | PC, bladder cancer, UTUC, kidney cancer, testicular cancer | 2019 pre-pandemic versus 2020 pandemic | 880 with PC; 414 pre-pandemic versus 466 pandemic | No changes in ISUP and lymph node involvement in RP result, no delay between diagnosis and RP (105 vs. 102 days). More MRI-fusion biopsy (84% vs. 75%, p = 0.002), higher proportion of ISUP 3/4 (p = 0.001), lower rate of pT3 and positive surgical margins when RPs were performed |
| Kalemci [21] (2022) | Turkey | Retrospective | PC, kidney cancer, bladder cancer testicular cancer | July 2018 to December 2019 versus July 2020 to December 2020 | 310 with PC; 198 pre-pandemic versus 112 pandemic | No difference in terms of PSA, ISUP grade, positive surgical margins, pathological stage. Higher rate of pN1+ patients (12.5% vs. 4.5%, p = 0.026) |
| Barreras [22] (2022) | Spain | Retrospective | PC | January 2019 to December 2019 versus January 2020 to December 2020 | 787; 497 pre-pandemic versus 290 pandemic | No difference in terms of positive biopsies, waiting time till surgery, BCA at 1 year. Higher PSA levels (14.3 vs. 9.9, p = 0.011), more patients with metastatic disease (7.3% vs. 1.9%, p = 0.009), more pT3 tumors (37.2% vs. 27.2%, p = 0.08), higher rate of positive surgical margins (48.6% vs. 29.3%, p = 0.027) |
| Andrade [23] (2023) | Brazil | Retrospective | PC, only patients with RP | November 2018 to February 2020 versus March 2020 to June 2021 | 226; 88 pre-pandemic versus 138 pandemic | No difference in mean age, BMI, ASA pT3, ISUP grading. Longer time between medical consultation and surgery (124 vs. 107 days, p < 0.0001) and between biopsy and surgery (198.5 vs. 228 days, p = 0.013) |
| Borz [24] (2024) | Romania | Retrospective | PC, only patients with RP | 2016 to March 16, 2020 versus March 16, 2020–2022 | 423; 289 pre-pandemic versus 134 pandemic | No changes in ISUP, stable BCR rates for 21-month follow-up. Nonsignificant trend to higher PSA levels, nonsignificant increase in lymph node involvement |
| First author (year) . | Location . | Type of study . | Type of malignancy included . | Comparison period . | Patients included . | Main result . |
|---|---|---|---|---|---|---|
| Moschovas [19] (2021) | USA | Retrospective | PC, only patients with RP | March 1 to May 25, 2020, no comparison period | 147 | No patient with COVID-19 infection after RARP, 2 patients were rescheduled to positive COVID-19 test. RARP is safe and feasible during the pandemic |
| Oderda [20] (2021) | Italy | Retrospective | PC, bladder cancer, UTUC, kidney cancer, testicular cancer | 2019 pre-pandemic versus 2020 pandemic | 880 with PC; 414 pre-pandemic versus 466 pandemic | No changes in ISUP and lymph node involvement in RP result, no delay between diagnosis and RP (105 vs. 102 days). More MRI-fusion biopsy (84% vs. 75%, p = 0.002), higher proportion of ISUP 3/4 (p = 0.001), lower rate of pT3 and positive surgical margins when RPs were performed |
| Kalemci [21] (2022) | Turkey | Retrospective | PC, kidney cancer, bladder cancer testicular cancer | July 2018 to December 2019 versus July 2020 to December 2020 | 310 with PC; 198 pre-pandemic versus 112 pandemic | No difference in terms of PSA, ISUP grade, positive surgical margins, pathological stage. Higher rate of pN1+ patients (12.5% vs. 4.5%, p = 0.026) |
| Barreras [22] (2022) | Spain | Retrospective | PC | January 2019 to December 2019 versus January 2020 to December 2020 | 787; 497 pre-pandemic versus 290 pandemic | No difference in terms of positive biopsies, waiting time till surgery, BCA at 1 year. Higher PSA levels (14.3 vs. 9.9, p = 0.011), more patients with metastatic disease (7.3% vs. 1.9%, p = 0.009), more pT3 tumors (37.2% vs. 27.2%, p = 0.08), higher rate of positive surgical margins (48.6% vs. 29.3%, p = 0.027) |
| Andrade [23] (2023) | Brazil | Retrospective | PC, only patients with RP | November 2018 to February 2020 versus March 2020 to June 2021 | 226; 88 pre-pandemic versus 138 pandemic | No difference in mean age, BMI, ASA pT3, ISUP grading. Longer time between medical consultation and surgery (124 vs. 107 days, p < 0.0001) and between biopsy and surgery (198.5 vs. 228 days, p = 0.013) |
| Borz [24] (2024) | Romania | Retrospective | PC, only patients with RP | 2016 to March 16, 2020 versus March 16, 2020–2022 | 423; 289 pre-pandemic versus 134 pandemic | No changes in ISUP, stable BCR rates for 21-month follow-up. Nonsignificant trend to higher PSA levels, nonsignificant increase in lymph node involvement |
PC, prostate cancer; RARP, robotic-assisted radical prostatectomy; RP, radical prostatectomy; UTUC, upper tract urothelial carcinoma; BCR, biochemical recurrence.
Conclusion
In our analysis regarding the diagnosis and treatment of PC, we could not demonstrate any shifts or delays due to the pandemic. However, it will take several more years to demonstrate the true impact of the COVID-19 pandemic on the diagnosis and treatment of PC due to the natural course of the disease.
Statement of Ethics
This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All members of the research team committed themselves to the confidentiality of the information provided as well as to data protection and are subject to medical confidentiality. Ethical approval was not required for this study in accordance with local/national guidelines. Written informed consent to participate in the study was not required in accordance with local/national guidelines.
Conflict of Interest Statement
Immanuel A. Oppolzer, Bayer, Bristol Myers Squibb, GlaxoSmithKline, Ipsen, Janssen, Medac, Merck, MSD, and Pfizer: travel/accommodation/expenses. Apogepha and Bayer: stock ownership. Bayer, Merck & Co, AstraZeneca, Marco J. Schnabel, Ipsen, MSD, QED Therapeutics Inc., and Maximilian Burger: advisory board members. Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck & Co, Novartis, and Pfizer: honoraria. Photocure: honoraria. Janssen, Michael Gierth, Selma Hammer, Maximilian Haas, Christopher Goßler, Hannah Zilles, and Maximilian Mueller have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Immanuel A. Oppolzer: project development, data analysis, data management, and manuscript writing. Marco J. Schnabel: project development, data management, and data analysis. Selma Hammer, Maximilian Haas, Christopher Goßler, Maximilian R. Mueller, and Hannah Zilles: data collection and data management. Maximilian Burger: data management and resources. Michael Gierth: project development and data management.
Data Availability Statement
The data used to support the findings of this study are included in this article. Further inquiries can be directed to the corresponding author.