Abstract
Introduction: Despite the prospective randomized controlled JAVELIN Bladder 100 trial, no real-world evidence exists regarding tumor characteristics, adverse events (AEs), and survival of avelumab maintenance (AVM)-treated patients with partial/complete response or stable disease after previous platinum-based chemotherapy for advanced/metastatic urothelial carcinoma (mUC). Methods: We relied on our institutional database to identify mUC patients who received AVM between January, 2021 and December, 2023. The main outcomes consisted of overall survival (OS) and progression-free survival (PFS) and were computed by Kaplan-Meier estimates. Stratification was performed according to programmed death ligand 1 (PD-L1) status. Results: Overall, 24 AVM patients were identified at a median age of 71 (interquartile range [IQR]: 67–76) years, of which 67% were males. Of these, 63%, 21%, and 17% received AVM therapy for bladder cancer and upper tract urothelial carcinoma or both, respectively. PD-L1 status was positive in 45% of patients. During AVM treatment, AEs were observed in 33% of patients; however, they were limited to ≤2 grade AEs. At a median follow-up of eight (IQR 4–20) months, 71% of patients had progressed under AVM with median PFS of 6.2 months (confidence interval [CI]: 3.2–18.2). Median OS was 13.4 (CI: 6.9 – not reached [NR]) months. One-year OS after AVM was 52%. In PD-L1-positive patients, median PFS and OS were 6.4 (CI: 2.7 – NR) months and 13.4 (CI: 7.7 months – NR), respectively. Conclusion: AVM is associated with moderate AE rates. Despite similarities in baseline characteristics compared to trial-selected JAVELIN Bladder 100 mUC patients, AVM resulted in longer/similar PFS but significantly shorter OS in real-world setting.
Introduction
Bladder cancer is the tenth most common cancer worldwide, and urothelial cancer is the most common histological subtype of bladder cancer and upper tract urothelial cancer and accounts for about 90% of all cases [1‒5]. Its treatment becomes more difficult as the disease progresses, and in patients with locally advanced/metastatic urothelial carcinoma (mUC), 5-year survival rate is still only 5% [6‒8]. Currently, combination of chemotherapy with platinum-based therapies is the first-line standard of care (SOC) for mUC, but severe side effects limit the long-term use of these chemotherapy regimens, and most patients experience disease progression within approximately 9 months of initial response. Moreover, the median overall survival (OS) is limited to 14–15 months and 9–10 months for cisplatin-based regimens and carboplatin-based regimens, respectively [9].
With the clinical approval of immune checkpoint inhibitors, new treatment options are now available for mUC for the first time in many years. By blocking the interaction of programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) receptors with therapeutic antibodies, the suppression of the immune response directed against the tumor is to be reversed. Good response rates can be achieved in bladder cancer patients with PD-1/PD-L1 inhibitors with low toxicity [10‒15].
For mUC patients’ combination of chemotherapy with platinum-based regimens followed by maintenance therapy with avelumab (AVM) has become the SOC [16]. The results of the prospective randomized controlled phase III JAVELIN Bladder 100 trial showed that AVM significantly prolonged progression-free survival (PFS) and OS compared to SOC, with a median OS of 23.8 months and favorable safety profile. Moreover, AVM also significantly prolonged PFS and OS in the subgroup of PD-L1-positive mUC patients in a more pronounced fashion, relative to SOC [12, 14].
Despite several reviews placing these new AVM data in the context of the current mUC literature, there are no further studies or real-world data on survival rates, safety profiles, and adverse events (AEs) [17, 18]. We addressed this knowledge gap and hypothesized that in clinical practice differences between AVM patients exist regarding of baseline characteristics and PFS and OS outcomes in unselected and PD-L1-positive mUC patients, relative to trial-selected JAVELIN Bladder 100 patients.
Methods
Patient Cohort
For the current study, all patients with mUC were retrospectively included from the database of the Department of Urology at the Goethe University Hospital in Frankfurt, Germany, following prior ethics approval (approval number SUG-4-2019). All included patients had previously received platinum-based chemotherapy with radiologically proven response or stable disease.
Inclusion period of the study was from January, 2021 to December, 2023. All patient and tumor characteristics, as well as oncological information were taken anonymously from the patient files. Patients were treated with AVM until disease progression or unacceptable toxicity. Exclusion criteria consisted of patients refusing AVM therapy after chemotherapy (n = 1).
AVM Therapy
The dose of avelumab as monotherapy was 800 mg or 10 mg/kg body weight intravenous infusion every 2 weeks and was administered intravenously over 60 min. The patients received an antihistamine and paracetamol as premedication. Prior to treatment, a laboratory sample was taken, and possible side effects were investigated. Staging by means of computer tomography or magnet resonance imaging was performed every 3 months during AVM therapy. In the event of clinical and/or radiological progression, AVM therapy was discontinued.
Statistical Analysis and Study Endpoints
The descriptive data analyses included frequency distributions for categorical variables, as well as median calculations including interquartile ranges (IQR) and overall distribution (range) for continuous variables. The radiological findings before and during treatment were used as the short-term oncological treatment response. For the purpose of the side effect profile of AVM therapy, therapy interruptions as well as the frequency and severity of side effects were evaluated and categorized according to common terminology criteria for adverse events (CTCAE) [19].
Kaplan-Meier estimates were used to predict primary study outcome of OS and PFS. Sensitivity analyses were performed for PD-L1-positive AVM patients. All analyses were performed using the statistical software R (version 4.1.2).
Results
A total of 24 patients with mUC in the period from January 2021 to December 2023 were included in the present study who received AVM according to the JAVELIN Bladder 100 trial [12]. All patients had received platinum-based chemotherapy with radiological evidence of stable disease progression or response to therapy prior to AVM therapy. Median follow-up of the current study was 8 months.
Patient and Tumor Characteristics
The median age of the included AVM patients was 71 (IQR: 66–76; Table 1) years. The proportion of males was 67% (n = 16) with an Eastern Cooperative Oncology Group (ECOG) status of 0–1 in 92% of cases. Overall, eight percent of patients were classified as ECOG 2 status. mUC of the bladder was present in 63% (n = 15), while the upper urinary tract was primarily affected in 21% (n = 5) of AVM patients. mUC as a primary tumor in both the bladder and the upper urinary tract was present in 17% (n = 4). Detailed laboratory results of all included patients prior to AVM and at time of end of treatment are displayed in Table 1.
Descriptive characteristics of 24 patients with advanced or mUC treated with avelumab maintenance (AVM) between January 2021 and December 2022
| Variable . | . | N = 24 . |
|---|---|---|
| Age at start AVM, years | Median (IQR) | 71 (66–76) |
| Sex | Male | 16 (66.7) |
| Side of primary tumor | Bladder | 15 (62.5) |
| UTUC | 5 (20.8) | |
| Both | 4 (16.7) | |
| ECOG status | 0 | 8 (33.3) |
| 1 | 14 (58.3) | |
| 2 | 2 (8.3) | |
| Cycles AVM | Median (IQR) | 12 (5–16) |
| CRP prior AVM, mg/dL | Median (IQR) | 0.7 (0.2–2.2) |
| CRP at PD, mg/dL | Median (IQR) | 4.9 (2.0–12.2) |
| Hemoglobin prior AVM, g/dL | Median (IQR) | 10.1 (9.1–11.1) |
| Hemoglobin at PD, g/dL | Median (IQR) | 11.2 (10.2–12.7) |
| Thrombocytes prior AVM, per nL | Median (IQR) | 201 (171–394) |
| Thrombocytes at PD, per nL | Median (IQR) | 262 (197–315) |
| Leucocytes prior AVM, per µL | Median (IQR) | 6.1 (5.0–7.4) |
| Leucocytes at PD, per µL | Median (IQR) | 8.3 (6.9–12.4) |
| Neutrophiles prior AVM, per µL | Median (IQR) | 3.1 (2.1–3.9) |
| Neutrophiles at PD, per µL | Median (IQR) | 7.9 (4.7–9.9) |
| Creatinine prior AVM, mg/dL | Median (IQR) | 1.2 (1.0–1.4) |
| Creatinine at PD, mg/dL | Median (IQR) | 1.3 (1.0–1.8) |
| LDH prior AVM, U/L | Median (IQR) | 241 (224–287) |
| LDH at PD, U/L | Median (IQR) | 253 (194–316) |
| ALT prior AVM, U/L | Median (IQR) | 18 (15–22) |
| ALT at PD, U/L | Median (IQR) | 19 (15–25) |
| GGT prior AVM, U/L | Median (IQR) | 53 (40–101) |
| GGT at PD, U/L | Median (IQR) | 68 (30–109) |
| Local treatment | None | 9 (37.5) |
| Cystectomy | 9 (37.5) | |
| Nephroureterectomy | 4 (16.7) | |
| Other | 3 (8.3) | |
| pT stage at local treatment | ≤pT2 bladder/UTUC | 8 (33.4) |
| pT3 bladder/UTUC | 6 (24.7) | |
| pT4 bladder/UTUC | 4 (16.7) | |
| Unknown | 4 (16.7) | |
| pN stage | N+ | 7 (29.2) |
| Metastatic disease | Primary | 14 (58.3) |
| Secondary | 10 (41.7) | |
| Metastatic side at start AVM | Nodal | 5 (20.8) |
| Bone | 4 (16.7) | |
| Visceral | 15 (62.5) | |
| Cycles first-line chemotherapy | Median (IQR) | 4 (3–5) |
| First-line chemotherapy regime | Gemcitabine/carboplatin | 7 (29.2) |
| Gemcitabine/cisplatin | 12 (50.0) | |
| Gemcitabine/cis or carboplatin | 3 (12.5) | |
| Other platin-based regimes | 2 (8.3) | |
| PD-L1 status | Negative | 4 (16.7) |
| Positive | 11 (45.8) | |
| Unknown | 9 (37.5) | |
| If PD-L1 positive, % | Median (IQR) | 10 (6–22) |
| Variable . | . | N = 24 . |
|---|---|---|
| Age at start AVM, years | Median (IQR) | 71 (66–76) |
| Sex | Male | 16 (66.7) |
| Side of primary tumor | Bladder | 15 (62.5) |
| UTUC | 5 (20.8) | |
| Both | 4 (16.7) | |
| ECOG status | 0 | 8 (33.3) |
| 1 | 14 (58.3) | |
| 2 | 2 (8.3) | |
| Cycles AVM | Median (IQR) | 12 (5–16) |
| CRP prior AVM, mg/dL | Median (IQR) | 0.7 (0.2–2.2) |
| CRP at PD, mg/dL | Median (IQR) | 4.9 (2.0–12.2) |
| Hemoglobin prior AVM, g/dL | Median (IQR) | 10.1 (9.1–11.1) |
| Hemoglobin at PD, g/dL | Median (IQR) | 11.2 (10.2–12.7) |
| Thrombocytes prior AVM, per nL | Median (IQR) | 201 (171–394) |
| Thrombocytes at PD, per nL | Median (IQR) | 262 (197–315) |
| Leucocytes prior AVM, per µL | Median (IQR) | 6.1 (5.0–7.4) |
| Leucocytes at PD, per µL | Median (IQR) | 8.3 (6.9–12.4) |
| Neutrophiles prior AVM, per µL | Median (IQR) | 3.1 (2.1–3.9) |
| Neutrophiles at PD, per µL | Median (IQR) | 7.9 (4.7–9.9) |
| Creatinine prior AVM, mg/dL | Median (IQR) | 1.2 (1.0–1.4) |
| Creatinine at PD, mg/dL | Median (IQR) | 1.3 (1.0–1.8) |
| LDH prior AVM, U/L | Median (IQR) | 241 (224–287) |
| LDH at PD, U/L | Median (IQR) | 253 (194–316) |
| ALT prior AVM, U/L | Median (IQR) | 18 (15–22) |
| ALT at PD, U/L | Median (IQR) | 19 (15–25) |
| GGT prior AVM, U/L | Median (IQR) | 53 (40–101) |
| GGT at PD, U/L | Median (IQR) | 68 (30–109) |
| Local treatment | None | 9 (37.5) |
| Cystectomy | 9 (37.5) | |
| Nephroureterectomy | 4 (16.7) | |
| Other | 3 (8.3) | |
| pT stage at local treatment | ≤pT2 bladder/UTUC | 8 (33.4) |
| pT3 bladder/UTUC | 6 (24.7) | |
| pT4 bladder/UTUC | 4 (16.7) | |
| Unknown | 4 (16.7) | |
| pN stage | N+ | 7 (29.2) |
| Metastatic disease | Primary | 14 (58.3) |
| Secondary | 10 (41.7) | |
| Metastatic side at start AVM | Nodal | 5 (20.8) |
| Bone | 4 (16.7) | |
| Visceral | 15 (62.5) | |
| Cycles first-line chemotherapy | Median (IQR) | 4 (3–5) |
| First-line chemotherapy regime | Gemcitabine/carboplatin | 7 (29.2) |
| Gemcitabine/cisplatin | 12 (50.0) | |
| Gemcitabine/cis or carboplatin | 3 (12.5) | |
| Other platin-based regimes | 2 (8.3) | |
| PD-L1 status | Negative | 4 (16.7) |
| Positive | 11 (45.8) | |
| Unknown | 9 (37.5) | |
| If PD-L1 positive, % | Median (IQR) | 10 (6–22) |
IQR, interquartile range; ECOG, Easter Cooperative Oncology Group; CRP, C-reactive protein; PD, progressive disease; LDH, lactate dehydrogenase; ALT, alanine transaminase; GGT, gamma-glutamyltransferase; PD-L1, programmed death ligand 1.
Regarding PD-L1 status, 46% (n = 11) patients showed positive status with a median positivity of the tumor sample of 10% (IQR: 6–22%). Chemotherapy with cisplatin was given to 50% of AVM patients. Other regimes with cisplatin or carboplatin were administered in the remaining 50% of AVM patients. On median, the patients received four (IQR: 3–5) cycles of platinum-containing chemotherapy. In the initial staging before the start of AVM therapy, 63% (n = 15) of patients showed visceral metastases.
Treatment Response
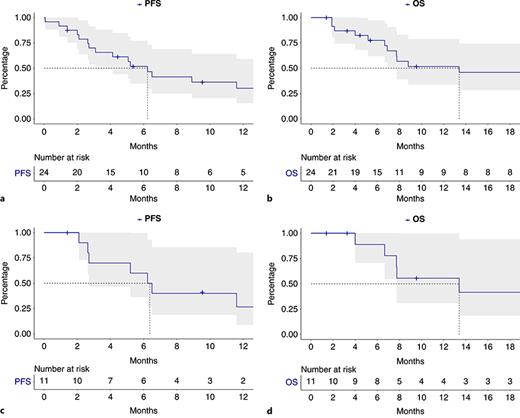
After median administration of 12 (IQR: 5–16) cycles of AVM at time of censoring, median PFS of AVM patients was 6.2 (95% confidence interval [CI]: 3.1–18.0) months (Fig. 1a). Median OS of AVM patients was 13.4 (CI: 6.9 – not reached [NR]) months (Fig. 1a). Overall, 1-year OS rate was 52% with AVM.
Progression-free survival (PFS, a) and overall survival (OS, b) of all advanced or metastatic urothelial carcinoma (mUC) patients receiving AVM therapy and PFS (c), as well as OS (d) of all PD-L1 status-positive mUC patients.
Progression-free survival (PFS, a) and overall survival (OS, b) of all advanced or metastatic urothelial carcinoma (mUC) patients receiving AVM therapy and PFS (c), as well as OS (d) of all PD-L1 status-positive mUC patients.
AEs and Safety Management
During the administration of AVM for mUC, a total of eight patients (33%) experienced AEs (Table 2). Of these, four patients showed moderate AEs (grade II, 17%), and 4 patients had mild AEs (grade I, 17%). There were no serious or higher-grade side effects/AEs (≥grade III). Treatment with AVM was discontinued in 5 patients (21%), and 1 patient received blood transfusion under AVM therapy. After mUC disease progression, subsequent therapy regimes are displayed in Table 2 and contained enfortumab vedotin, erdafitinib, chemotherapy, or pembrolizumab.
Adverse events (AEs) and subsequent therapy of 24 patients with advanced or mUC treated with avelumab maintenance (AVM) between January 2021 and December 2022
| Variable . | . | N = 24 . |
|---|---|---|
| Transfusion under AVM | 1 (4.5) | |
| AE | 8 (33.3) | |
| AE | Grade I | 4 (16.7) |
| Grade II | 4 (16.7) | |
| Discontinuation of AVM due to AE | 5 (20.8) | |
| Therapy after AVM | Enfortumab vedotin | 4 (16.7) |
| Erdafitinib | 1 (4.2) | |
| Gemcitabine/carboplatin | 1 (4.2) | |
| Paclitaxel | 2 (8.3) | |
| Pembrolizumab | 2 (8.3) | |
| Vinflunine | 1 (4.2) |
| Variable . | . | N = 24 . |
|---|---|---|
| Transfusion under AVM | 1 (4.5) | |
| AE | 8 (33.3) | |
| AE | Grade I | 4 (16.7) |
| Grade II | 4 (16.7) | |
| Discontinuation of AVM due to AE | 5 (20.8) | |
| Therapy after AVM | Enfortumab vedotin | 4 (16.7) |
| Erdafitinib | 1 (4.2) | |
| Gemcitabine/carboplatin | 1 (4.2) | |
| Paclitaxel | 2 (8.3) | |
| Pembrolizumab | 2 (8.3) | |
| Vinflunine | 1 (4.2) |
Discussion
For immunotherapy treatment-naïve patients, combination chemotherapy with platinum-based regimens followed by maintenance therapy with avelumab has become the SOC for mUC [2]. The prospective randomized controlled phase III JAVELIN Bladder 100 trial showed that patients with mUC whose disease had not progressed on first-line platinum-based chemotherapy had significantly longer OS on maintenance treatment with avelumab than on best supportive care alone; this result was true for both the overall population and the PD-L1-positive population [12, 14]. Avelumab was therefore approved by the FDA (2020) and the EMA (2021) for the treatment of patients with mUC in whom platinum-based chemotherapy in the first line has not resulted in disease progress. In addition, maintenance treatment with avelumab after platinum-based chemotherapy is now recommended in the various guidelines (recommendation grade: I, A) [2, 16].
The data available for AVM in mUC are currently provided from clinical trials. However, these results allow only limited insight into the real-world efficacy (i.e., effectiveness measured as PFS or OS) and safety of AVM in routine clinical practice. Therefore, real-world data and evidence are crucial to understand the extent of clinical benefit, impact on patient-reported outcomes, tolerability of therapy, and management of toxicities in patients with mUC treated with AVM as part of maintenance therapy. In order to address these unmet needs, we conducted the current study and made several important observations.
First, compared to trial-selected JAVELIN Bladder 100 AVM patients, our data show a longer to similar median PFS (6.2 months vs. 5.5 months) but significantly shorter OS (13.4 months vs. 23.8 months) of all treated AVM mUC patients. It is additionally of note that despite the median OS with AVM of 23.8 months in the JAVELIN Bladder 100 trial, the control arm had also a slightly longer median OS with 15.0 months than our AVM cohort, resulting in a hazard ratio of 0.76 (CI: 0.63–0.91; p = 0.0036) in favor of AVM therapy. Moreover, 1-year survival rate was 79.1% in the AVM group and 60.4% in the control group, while in our real-world evidence AVM cohort, only 52% OS after 1 year was observed [12, 14].
In subgroup-specific analyses for PD-L1-positive AVM mUC patients, median PFS was 6.4 months and median OS was 13.4 months at a median follow-up of 8 months, while JAVELIN Bladder 100 patients with positive PD-L1 status harbored longer median PFS (7.5 months) and median OS (30.9 months) at median follow-up of 38 months [14].
These differences in shorter OS outcomes may be explained by differences in patients and tumor characteristics in clinical real-world setting. For example, the median age of our included AVM patients was 71 years and slightly older than the population of the JAVELIN Bladder 100 trial (median age 68 years). Moreover, our AVM patient population was also mostly categorized as ECOG 0–1 at the start of avelumab therapy. However, 8% of our AVM patient collective were classified as ECOG 2, while no ECOG 2 patients were included in the JAVELIN Bladder 100 trial [12].
Additionally, some of our patients would not have fulfilled the inclusion criteria of the JAVELIN 100 Bladder trial with their initial laboratory values prior to AVM therapy. For example, hemoglobin was low with a median value of 10.1 g/dL (IQR 9.1–11.1 g/dL). Moreover, the proportion of patients with visceral metastases was significantly higher in our group with 63% compared to 54%, which may have also resulted in shorter OS compared to JAVELIN 100 Bladder trial patients. However, our AVM patient cohort is limited by sample size; thus, our data are of descriptive nature and no comparative statistical analyses were performed, and advanced statistical analyses such as multivariable regression models could be fitted, as performed in previous publications regarding bladder cancer or upper tract urothelial cancer [20‒22]. An interesting approach would be to compare the PFS and OS of patients with AVM with patients undergoing immunotherapy in second-line therapy in progress with platinum-containing chemotherapy.
Moreover, our data show that AVM is an effective and safe treatment option for patients with mUC. The rate of AEs was therefore low to moderate and easily manageable. Since immunotherapy is now so firmly implemented in our daily clinical practice, it can be assumed that the side effects can be recognized and counteracted at a very early stage, thanks to our longer experience handling immunotherapy in urologic oncology. Compared to the JAVELIN Bladder 100 trial where grade III AEs according to CTCAE occurred in 47% of AVM patients, no patient harbored grade III AE in our AVM cohort [12]. However, these comparisons need to be interpreted in the light of the small patient AVM cohort and the retrospective character of our study.
Due to the fact that enfortumab vedotin was approved for third-line treatment during our study period, the follow-up therapies are rather heterogenous [23]. It therefore remains to be seen which follow-up therapy will be the most successful in the real-world setting since these therapies also influence OS significantly. Additionally, with new upcoming promising results from the Checkmate 901 (nivolumab + gemcitabine/cisplatin) and EV 302/keynote-A39 study (enfortumab vedotin + pembrolizumab) in first-line treatment for mUC, the further role of AVM needs to be reevaluated [24, 25].
Moreover, the present study has some further limitations and does not claim to be a fundamental evaluation of the oncological response of AVM. In view of the small patient cohort and the relatively short follow-up, the study is rather intended to present initial clinical applicability data.
Conclusion
mUC patients in real-world setting share similarities with trial-selected AVM patients. However, important differences such as age or performance status or occurrence of visceral metastases may determine shorter median OS than in trial-selected patients. However, in clinical practice, AVM is safe with low rates of AEs.
Statement of Ethics
This study protocol was reviewed and the need for approval and written informed consent was waived by the Ethics Committee of the Goethe University Hospital Frankfurt, approval number SUG-4-2019.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received for the current study.
Author Contributions
Banek Severine and Wenzel Mike: manuscript writing/editing, protocol/project development, and data analysis; Lauer Benedikt: data collection and management; Le Chi Quynh, Hoeh Benedikt, Koll Florestan, Cano Garcia Cristina, and Humke Clara: data analysis and protocol development; Köllermann Jens: data analysis, protocol development, and supervision; K.H. Chun Felix: manuscript writing/editing and supervision; Kosiba Marina: manuscript writing/editing; and A. Kluth Luis: protocol/project development and manuscript writing/editing.
Additional Information
Séverine Banek and Mike Wenzel share the first authorship.
Data Availability Statement
The data that support the findings of this study are not publicly available due to containing information that could compromise the privacy of research participants but are available from the corresponding author (M.W.) upon reasonable request.