Abstract
Introduction: We evaluated the effectiveness and safety profile of the tyrosine kinase inhibitor sunitinib in patients with advanced or metastatic renal cell carcinoma (a/mRCC) in a real-world setting. Methods: We analyzed data of adult a/mRCC patients treated with sunitinib. Data were derived from the German non-interventional post-approval multicenter STAR-TOR registry (NCT00700258). Progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were evaluated using descriptive statistics and survival analyses for the entire cohort and patient subgroups. Results: A total of 116 study sites recruited 702 patients treated with sunitinib (73.1% male; median age 68.0 years; median Karnofsky index 90%) between November 2010 and May 2020. The most frequent histological subtype was clear cell RCC (81.6%). Sunitinib was administered as first-line treatment in 83.5%, as second line in 11.7%, and as third line or beyond in 4.8% of the patients. Drug-related AEs and serious AEs were reported in 66.3% and 13.9% of the patients, respectively (most common AE: gastrointestinal disorders; 39.7% of all patients). Conclusions: This study adds further real-world evidence of the persisting relevance of sunitinib for patients with a/mRCC who cannot receive or tolerate immune checkpoint inhibitors. The study population includes a high proportion of patients with unfavorable MSKCC poor-risk score, but shows still good PFS and OS results, while the drug demonstrates a favorable safety profile. The STAR-TOR registry is also registered in the database of US library of medicine (NCT00700258).
Introduction
Treatment recommendations for advanced or metastatic renal cell carcinoma (a/mRCC) are divided into two paths based on patient characteristics. The suitable patients receive combination therapies with immune checkpoint inhibitors (ICIs), whereas for the remainder sunitinib and pazopanib are recommended plus cabozantinib in case of International Metastatic RCC Database Consortium (IMDC) intermediate and poor-risk status [1]. Of these therapeutic options, sunitinib is the most extensively studied drug and as such was regarded as the gold standard in first-line a/mRCC treatment for over 10 years. Therefore, sunitinib was the reference medication in most of the studies which assessed first-line ICI combination therapies in the last several years.
Sunitinib has been EMA-approved in July 2006 for pretreated patients with a/mRCC after failure of interferon-α or interleukin-2 therapy. The phase II registrational study demonstrated an overall response rate of 34% and a progression-free survival (PFS) of 8.3 months [2]. In the pivotal phase III trial, sunitinib was compared to interferon-α and demonstrated a striking PFS of 11 months for sunitinib versus 5 months for interferon-α (p < 0.001) [3]. Thus, in 2007 the marketing authorization of sunitinib was amended to include a/mRCC patients regardless of line of therapy.
Even though nowadays this study must be considered as a mere historic event, the survival benefit for patients that sunitinib brought to the treatment landscape was tremendous while treatment-related adverse events (AEs) seemed to be moderate. Not only was sunitinib intensely examined in prospective clinical trials but there was additional real-world evidence generated over the years. Beginning with the publication of the expanded access trial in 2009 which included 4,543 patients, several observational studies were to follow [4]. Nevertheless, the STAR-TOR registry represents one of the largest real-world data sets describing not only effectivity but also tolerability data for the use of sunitinib in the routine clinical setting.
Material and Methods
Our data originate from the prospective STAR-TOR registry maintained by Pfizer Pharma GmbH, Berlin, Germany, and were collected in accordance with the Declaration of Helsinki in its most recent version. The STAR-TOR registry received prior approval by the Ethics Committee of Münster University Hospital in 2007 (Nr.2007-484-f-S) and is also registered in the database of the US library of medicine (NCT00700258). The registry evaluates the treatment with (a) axitinib, (b) sunitinib, or (c) temsirolimus in a routine clinical setting for its effectiveness, tolerability, and safety. Target diseases are a/mRCC, recurrent or refractory mantle cell lymphoma, and gastrointestinal stromal tumors. Additional details regarding the STAR-TOR registry have been published previously [5‒9].
Study Cohort
The here-presented analyses include adult patients with histologically confirmed a/mRCC treated with sunitinib monotherapy at the STAR-TOR study centers between November 2010 and May 2020. All patients gave written informed consent for enrollment in the STAR-TOR registry. All study centers were located in Germany.
Sunitinib Administration
The standard dosage of the orally administered sunitinib was 50 mg daily for 28 days, followed by a 14-day break (4/2 schedule). Treatment lines of sunitinib included any line, of which up to six lines of systemic therapy were documented.
Variables
The analyses included demographic variables and tumor variables. The MSKCC score was defined as the risk categorizing tool in the statistical analysis plan of the registry. As documentation of laboratory values was optional in this non-interventional study, the MSKCC score could not be calculated for every patient [10].
Serum lactate dehydrogenase (LDH) levels were measured by local laboratories. The MSKCC score for treatment-naïve patients defines a LDH concentration of 1.5 times the upper level of normal as a risk factor. Accordingly, “high LDH” was assumed when the serum LDH level exceeded 300 U/L [11].
Outcomes
This study evaluates three primary outcome measures: PFS, overall survival (OS), and AEs. Patients had their follow-up visits corresponding to the clinical routine at the participating sites in intervals of 8–12 weeks. At each follow-up visit, treatment status, response, tumor staging, laboratory values, and AEs were documented. All participating study centers received random monitoring visits ensuring correct patient assessment and data entry.
Treatment response was assessed according to RECIST 1.1 [12]. If RECIST evaluations were not routinely performed at the study sites, a physician’s assessment was accepted instead. Best response was determined by a physician at the final visit and entered in the case report form.
Definition for OS was time from the baseline visit at a participating STAR-TOR center until death from any cause. If documentation of death was not available, the subject was censored with the latest contact data. PFS was defined as the time from the baseline visit to documentation of disease progression or death.
AEs were reported by the study sites according to NCI-CTCAE criteria and the Medical Dictionary for Regulatory Activities (MedDRA) standard [13, 14]. For better comprehensibility of the analysis, AEs were grouped according to NCI-CTCAE standards.
Statistical Analyses
Categorial data are described as absolute frequencies and percentages. Continuous data are reported as median and range.
PFS and OS are visualized using Kaplan-Meier plots. The method of comparison for predefined subgroup analyses was log rank tests which were applied for treatment line, histologic subtype (clear cell RCC [ccRCC] vs. non-ccRCC), patient age (≤65 vs. >65 years), gender, MSKCC prognosis group (favorable vs. intermediate vs. poor), BMI (<25 vs. 25–30 vs. >30), and LDH levels (≤300 vs. >300).
Most performed analyses rely on univariate models as missing values, and patients with incomplete data would have demanded imputation of missing values or patient exclusion upon multivariate analyses what may have distorted our results. Possible correlations between nephrectomy and MSKCC prognosis group on PFS and OS were investigated using Cox regression models.
All statistical analyses were conducted using SAS software (version 9.4). The provided tests are two-sided and have to be interpreted purely exploratively.
The statistical analyses were performed by Dr. Thomas Fischer (Winicker Norimed GmbH, Nuremberg, Germany).
Results
Study Cohort
A total of 702 patients from 116 centers were included, of whom 659 completed their study participation until the final examination. Mean patients’ age was 68.0 years. A total of 513 patients were male (73.2%) with a median Karnofsky performance status score of 90%. Predominant tumor histology was ccRCC (81.6%). Risk score according to the MSKCC criteria was favorable in 12.6% of the patients, intermediate in 62.2%, and poor in 25.2%. In 85.3% of the patients, nephrectomy was performed, achieving a R0 status in 72.2% of the cases. For n = 568 patients, time point of nephrectomy was available: n = 309 RCC patients underwent nephrectomy before the diagnosis of metastases, n = 259 patients underwent nephrectomy after being diagnosed with metastatic disease, and n = 103 patients did not undergo any nephrectomy. All patients had metastatic disease upon inclusion in the registry. Table 1 details the characteristics of the study cohort.
Patient characteristics at baseline
| . | n . | . |
|---|---|---|
| Pts with final examination, n | 659 | |
| Age, years, median (range) | 702 | 68.0 (23.0–87.7) |
| Gender, male/female, % | 697 | 73.2/26.8 |
| Risk assessment according to MSKCC score, % | 460 | |
| Favorable risk | 12.6 | |
| Intermediate risk | 62.2 | |
| High risk | 25.2 | |
| Karnofsky performance status | 690 | |
| Median (range) | 90 (40–100) | |
| 100–90, % | 51.3 | |
| ≤80, % | 48.7 | |
| Histological subtype*, % | 702 | |
| Clear cell | 81.6 | |
| Papillary | 8.3 | |
| Chromophobe | 2.1 | |
| Collecting duct | 0.6 | |
| Unclassified and other | 7.8 | |
| Nephrectomy status, % | 702 | |
| Total nephrectomy | 76.6 | |
| Partial nephrectomy | 8.7 | |
| No nephrectomy | 14.7 | |
| Date of nephrectomy available | 568 | |
| Nephrectomy before diagnosis of metastatic disease | 54.4 | |
| Nephrectomy after diagnosis of metastatic disease | 45.6 | |
| Primary tumor resection, % | 601 | |
| R0 | 72.2 | |
| Incomplete | 27.8 | |
| Additional radiation therapy, % | 453 | |
| Yes | 10.6 | |
| No | 89.4 | |
| Number of metastatic sites, median (range) | 702 | 2 (0–6) |
| Location of metastases*, % | 702 | |
| Lung | 64.1 | |
| Lymph nodes | 41.2 | |
| Bone | 29.5 | |
| Liver | 17.9 | |
| Adrenal gland | 12.0 | |
| Contralateral kidney | 7.1 | |
| Central nervous system | 5.0 | |
| Other | 26.8 | |
| LDH level in serum, % | 564 | |
| ≤300 U/L | 81.9 | |
| >300 U/L | 18.1 | |
| BMI | 654 | |
| <25 | 30.7 | |
| 25–30 | 48.2 | |
| >30 | 21.1 |
| . | n . | . |
|---|---|---|
| Pts with final examination, n | 659 | |
| Age, years, median (range) | 702 | 68.0 (23.0–87.7) |
| Gender, male/female, % | 697 | 73.2/26.8 |
| Risk assessment according to MSKCC score, % | 460 | |
| Favorable risk | 12.6 | |
| Intermediate risk | 62.2 | |
| High risk | 25.2 | |
| Karnofsky performance status | 690 | |
| Median (range) | 90 (40–100) | |
| 100–90, % | 51.3 | |
| ≤80, % | 48.7 | |
| Histological subtype*, % | 702 | |
| Clear cell | 81.6 | |
| Papillary | 8.3 | |
| Chromophobe | 2.1 | |
| Collecting duct | 0.6 | |
| Unclassified and other | 7.8 | |
| Nephrectomy status, % | 702 | |
| Total nephrectomy | 76.6 | |
| Partial nephrectomy | 8.7 | |
| No nephrectomy | 14.7 | |
| Date of nephrectomy available | 568 | |
| Nephrectomy before diagnosis of metastatic disease | 54.4 | |
| Nephrectomy after diagnosis of metastatic disease | 45.6 | |
| Primary tumor resection, % | 601 | |
| R0 | 72.2 | |
| Incomplete | 27.8 | |
| Additional radiation therapy, % | 453 | |
| Yes | 10.6 | |
| No | 89.4 | |
| Number of metastatic sites, median (range) | 702 | 2 (0–6) |
| Location of metastases*, % | 702 | |
| Lung | 64.1 | |
| Lymph nodes | 41.2 | |
| Bone | 29.5 | |
| Liver | 17.9 | |
| Adrenal gland | 12.0 | |
| Contralateral kidney | 7.1 | |
| Central nervous system | 5.0 | |
| Other | 26.8 | |
| LDH level in serum, % | 564 | |
| ≤300 U/L | 81.9 | |
| >300 U/L | 18.1 | |
| BMI | 654 | |
| <25 | 30.7 | |
| 25–30 | 48.2 | |
| >30 | 21.1 |
*Multiple entries allowed.
LDH, lactate dehydrogenase.
Sunitinib Treatment
A total of 83.5% of the patients received sunitinib as first-line treatment, 11.7% as second line, and 4.8% as third line or beyond. Median treatment duration for all lines was 5.7 months. For 659 patients, data for treatment termination were available. Table 2 details the sunitinib treatment further.
Treatment characteristics
| . | n . | . |
|---|---|---|
| Sunitinib is used in, % | 702 | |
| First line | 83.5 | |
| Second line | 11.7 | |
| Third line | 3.1 | |
| Fourth line | 1.0 | |
| Fifth line and beyond | 0.7 | |
| Treatment duration | 642 | |
| Median, months (range) | 5.7 (0–83.6) | |
| Duration of survival follow-up | 367 | |
| Median, months (range) | 9.1 (0–91.5) | |
| Dose modifications | 576 | |
| Patients with dose changes, % | 67.7 | |
| Type of study end*, % | 659 | |
| Documentation until patient’s death | 14.7 | |
| Documentation discontinued | 9.0 | |
| Therapy discontinued | 76.3 | |
| Due to intolerability | 22.5 | |
| Due to progression | 75.7 | |
| Due to other reasons | 3.6 |
| . | n . | . |
|---|---|---|
| Sunitinib is used in, % | 702 | |
| First line | 83.5 | |
| Second line | 11.7 | |
| Third line | 3.1 | |
| Fourth line | 1.0 | |
| Fifth line and beyond | 0.7 | |
| Treatment duration | 642 | |
| Median, months (range) | 5.7 (0–83.6) | |
| Duration of survival follow-up | 367 | |
| Median, months (range) | 9.1 (0–91.5) | |
| Dose modifications | 576 | |
| Patients with dose changes, % | 67.7 | |
| Type of study end*, % | 659 | |
| Documentation until patient’s death | 14.7 | |
| Documentation discontinued | 9.0 | |
| Therapy discontinued | 76.3 | |
| Due to intolerability | 22.5 | |
| Due to progression | 75.7 | |
| Due to other reasons | 3.6 |
*More than one reason possible, for some pts no reason was given.
Sunitinib Treatment Response
Best response rates were reported for 669 patients with a clinical benefit rate of 63.8%. Complete response was reached in 6.0%, partial response in 24.8%, and stable disease in 33.0%, whereas 21.8% of the patients experienced disease progression. For 14.3% of the patients, no response was assessable. The median PFS for all patients was 6.7 months, and the median OS was 25.0 months.
Subgroup Analyses
Median survival was dependent on treatment line (1st vs. later line) with PFS 6.9 months (95% confidence interval [CI]: 6.1–8.3 months) versus 6.0 months (95% CI: 4.0–8.3 months, p = 0.29), and OS 25.6 months (95% CI: 22.2–28.9 months) versus 21.3 months (95% CI: 15.9–27.3 months, p = 0.11). Subgroup analysis results are displayed in Table 3. Median PFS and OS did not differ markedly between first, second, and third line and deteriorated clinically relevant not before sunitinib application in fourth and later lines.
Subgroup analyses of PFS and OS, months
| . | PFS . | OS . | ||
|---|---|---|---|---|
| n . | months . | n . | months . | |
| Median survival according to BMI and LDH | ||||
| All patients | 702 | 6.7 | 702 | 25.0 |
| BMI <25 | 201 | 4.8 | 201 | 20.9 |
| BMI 25–30 | 315 | 7.6 | 315 | 27.2 |
| BMI >30 | 138 | 11.1 | 138 | 27.8 |
| Patients with LDH ≤300 U/La | 462 | 7.7 | 462 | 27.3 |
| Patients with LDH >300 U/La | 102 | 4.4 | 102 | 9.8 |
| Median survival according to treatment line | ||||
| All patients | 702 | 6.7 | 702 | 25.0 |
| First-line patients | 586 | 6.9 | 586 | 25.6 |
| Second-line patients | 82 | 6.1 | 82 | 25.0 |
| Third-line patients | 22 | 6.9 | 22 | 24.3 |
| ≥Fourth-line patients | 12 | 4.9 | 12 | 8.6 |
| Median survival according to histology | ||||
| All patients | 702 | 6.7 | 702 | 25.0 |
| Clear cell patients | 573 | 7.4 | 573 | 25.7 |
| All other histologies | 129 | 5.0 | 129 | 20.9 |
| Median survival according to nephrectomy status | ||||
| No nephrectomy | 103 | 4.8 | 103 | 16.4 |
| Nephrectomy before diagnosis of metastatic disease | 309 | 8.0 | 309 | 28.0 |
| Nephrectomy after diagnosis of metastatic disease | 259 | 7.7 | 295 | 27.2 |
| . | PFS . | OS . | ||
|---|---|---|---|---|
| n . | months . | n . | months . | |
| Median survival according to BMI and LDH | ||||
| All patients | 702 | 6.7 | 702 | 25.0 |
| BMI <25 | 201 | 4.8 | 201 | 20.9 |
| BMI 25–30 | 315 | 7.6 | 315 | 27.2 |
| BMI >30 | 138 | 11.1 | 138 | 27.8 |
| Patients with LDH ≤300 U/La | 462 | 7.7 | 462 | 27.3 |
| Patients with LDH >300 U/La | 102 | 4.4 | 102 | 9.8 |
| Median survival according to treatment line | ||||
| All patients | 702 | 6.7 | 702 | 25.0 |
| First-line patients | 586 | 6.9 | 586 | 25.6 |
| Second-line patients | 82 | 6.1 | 82 | 25.0 |
| Third-line patients | 22 | 6.9 | 22 | 24.3 |
| ≥Fourth-line patients | 12 | 4.9 | 12 | 8.6 |
| Median survival according to histology | ||||
| All patients | 702 | 6.7 | 702 | 25.0 |
| Clear cell patients | 573 | 7.4 | 573 | 25.7 |
| All other histologies | 129 | 5.0 | 129 | 20.9 |
| Median survival according to nephrectomy status | ||||
| No nephrectomy | 103 | 4.8 | 103 | 16.4 |
| Nephrectomy before diagnosis of metastatic disease | 309 | 8.0 | 309 | 28.0 |
| Nephrectomy after diagnosis of metastatic disease | 259 | 7.7 | 295 | 27.2 |
BMI, body mass index; LDH, lactate dehydrogenase.
aExploratory analysis proved statistical significance with p < 0.0001.
Patients with ccRCC had longer median survival as patients with non-clear cell histologies (PFS: 7.4 vs. 5.0 months, p = 0.12; OS: 25.7 vs. 20.9 months, p = 0.14). Small subgroup numbers did not allow for further differentiation by histology. There was no clinically relevant difference in PFS between younger and older patients (≤65 years vs. >65 years: PFS: 6.8 vs. 6.7 months, p = 0.83; OS: 26.3 vs. 24.7 months, p = 0.92).
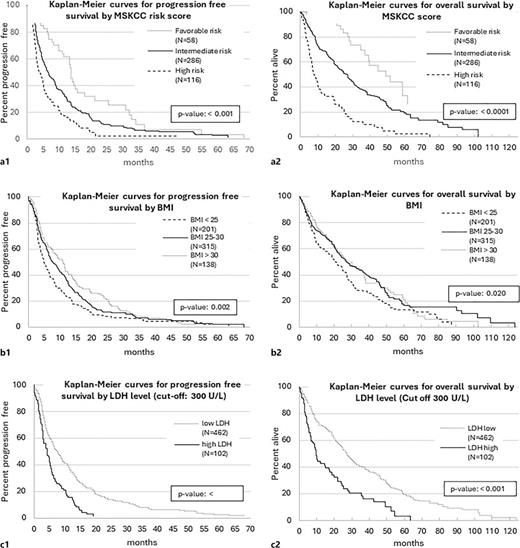
Similarly, no clinically relevant gender-specific differences were observed comparing male to female patients: PFS: 6.9 versus 6.2 months, p = 0.64; OS: 25.7 versus 22.8 months, p = 0.41. Worse prognosis according to MSKCC risk score was associated with shorter median PFS and OS (PFS from favorable to poor prognosis: 13.8, 6.5, 3.8 months, p < 0.0001; OS from favorable to poor prognosis: 49.3, 26.9, and 7.7 months, p < 0.0001).
Patients with BMI <25 had shorter PFS and OS than patients with BMI 25–30 and >30 (PFS: 4.8, 7.6, and 11.0 months, p = 0.002; OS: 20.9, 27.2, 27.8 months, p = 0.02). Upon stratification for baseline LDH serum levels at a cut-off of 300 U/L, PFS and OS markedly differed between patients with high versus low LDH (PFS: 4.4 vs. 7.7 months, p < 0.001, OS: 9.8 vs. 27.3 months, p < 0.001).
Figure 1 depicts the corresponding Kaplan-Meier curves. In addition, we stratified the patients by the time point of nephrectomy: of n = 568 patients with known nephrectomy status, median PFS and OS were compared for patients with nephrectomy before the diagnosis of metastatic disease versus for patients with already known metastatic disease at the time point of surgery versus patients without nephrectomy. Median PFS was 8.0 versus 7.7 versus 4.8 months and median OS 28.0 versus 27.7 versus 16.4 months, respectively. On multivariate analyses, the PFS and OS differences showed no statistical significance after adjusting for the MSKCC score (p > 0.05). No statistically significant interaction was evident between the MSKCC score and the nephrectomy status (p = 0.0.645 for PFS and p = 0.827 for OS, respectively). Given the low sample size of the MSKCC subgroups, no further stratifications were possible. Online supplementary Figure A (available at https://doi.org/10.1159/000536563) shows the corresponding Kaplan-Meier curves.
Kaplan-Meier curves for stratified survival analyses with PFS and OS for MSKCC Risk Score (a), BMI (b), and LDH level (c).
Kaplan-Meier curves for stratified survival analyses with PFS and OS for MSKCC Risk Score (a), BMI (b), and LDH level (c).
Adverse Events
Of 702 patients, 464 (66.3%) experienced at least one treatment-related AE. CTCAE grade III/IV events were reported for 97 (13.8%) patients. Table 4 details all AEs which were reported with a frequency of ≥2%, according to the MedDRA [13].
Treatment-related side effects in a total of 702 patients, incidence ≥2%
| . | Total . | Grade 3/4 . |
|---|---|---|
| Patients with AE, n (%) | 464 (66.1) | 97 (13.8) |
| Gastrointestinal disorders, % | 39.7 | 4.0 |
| General disorders, % | 29.5 | 3.8 |
| Skin and subcutaneous tissue disorders, % | 23.6 | 0.7 |
| Blood and lymphatic system disorders, % | 19.8 | 2.4 |
| Nervous system disorders, % | 18.7 | 0.9 |
| Investigations, % | 12.1 | 0.1 |
| Metabolism and nutrition disorders, % | 11.4 | 1.7 |
| Vascular disorders, % | 11.4 | 1.1 |
| Respiratory, thoracic, and mediastinal disorders, % | 7.7 | 1.4 |
| Musculoskeletal and connective tissue disorders, % | 5.4 | 0.3 |
| Infections and infestations, % | 4.8 | 2.0 |
| Endocrine disorders, % | 4.3 | 0.0 |
| Eye disorders, % | 2.6 | 0.0 |
| Renal and urinary tract disorders, % | 2.0 | 1.0 |
| . | Total . | Grade 3/4 . |
|---|---|---|
| Patients with AE, n (%) | 464 (66.1) | 97 (13.8) |
| Gastrointestinal disorders, % | 39.7 | 4.0 |
| General disorders, % | 29.5 | 3.8 |
| Skin and subcutaneous tissue disorders, % | 23.6 | 0.7 |
| Blood and lymphatic system disorders, % | 19.8 | 2.4 |
| Nervous system disorders, % | 18.7 | 0.9 |
| Investigations, % | 12.1 | 0.1 |
| Metabolism and nutrition disorders, % | 11.4 | 1.7 |
| Vascular disorders, % | 11.4 | 1.1 |
| Respiratory, thoracic, and mediastinal disorders, % | 7.7 | 1.4 |
| Musculoskeletal and connective tissue disorders, % | 5.4 | 0.3 |
| Infections and infestations, % | 4.8 | 2.0 |
| Endocrine disorders, % | 4.3 | 0.0 |
| Eye disorders, % | 2.6 | 0.0 |
| Renal and urinary tract disorders, % | 2.0 | 1.0 |
Discussion
Sunitinib was the standard therapeutic option in a/mRCC before the onset of ICI combination therapies and is even nowadays a well-established option for treatment for ICI unsuitable patients. Many trials investigated the effectiveness and safety of sunitinib, either at the beginning of the TKI era comparing it to previous options such as interferon-α or later on as the standard therapeutic option in modern combination therapy studies [3, 15]. Over time, sunitinib was compared to other TKIs in few phase II and phase III trials [16‒19]. Additionally, many real-world data sets have been published. The largest was the expanded access trial comprising 4,543 patients published by Gore et al. [4] in 2009, followed by numerous studies which were summarized by Moran et al. [20] in 2019. After 2019, to our knowledge, seven new real-world data sets were published.
As the number of publications directly evaluating sunitinib is large, Table 5 provides a comprehensive overview of the literature. Our study provides additional evidence and presents real-world data from the non-interventional post-approval multicenter STAR-TOR registry showing an overall OS of 25.0 months and a PFS of 6.7 months. Subgroup analyses confirmed worse prognosis score according to MSKCC, low BMI, and high LDH levels as negative predictive markers for sunitinib effectiveness. With respect to drug safety, AEs were observed in 66.3% of the patients (grade III/IV in 13.8%) with the most common AEs being gastrointestinal disorders (39.7% of all patients).
Sunitinib in clinical trials and real-world data sets – an overview
| Author, publication year . | Study type . | Pts receiving sunitinib, n . | Study drugs/comparisons . | Risk score used . | Risk stratification, % . | Percentage of ccRCC pts, % . | ORR, % . | PFS [months], median . | OS [months], median . | AE rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fav. . | int. . | poor . | CR . | PR . | SD . | PD . | all, % . | grade 3/4, % . | ||||||||
| STAR-TOR: this data set | Observational | 702 | Sunitinib or axitinib or temsirolimus | MSKCC | 12.6 | 62.2 | 25.2 | 81.6 | 6.0 | 24.8 | 33.0 | 21.8 | 6.7 | 25.0 | 66.3 | 13.9 |
| Motzer et al. [15] (2009) | Ph III RCT | 375 | Sunitinib versus IFN-α | MSKCC | 38 | 56 | 6 | 100 | 3 | 44 | 40 | 7 | 11.0 | 26.4 | N/R1 | N/R |
| Motzer et al. [45] (2012) | Ph II RCT | 1462 | Sunitinib only | MSKCC | 25 | 60 | 15 | 100 | 0 | 32 | 43 | N/R | 8.5 | 23.1 | N/R | N/R |
| Motzer et al. [16] (2013) | Ph III RCT | 548 | Sunitinib versus pazopanib | MSKCC (IMDC) | 27 (25) | 59 (26) | 9 (17) | 100 | <1 | 24 | 44 | 19 | 9.5 | 29.3 | 99 | 57/173 |
| Miyake et al. [21] (2014) | Observational | 102 | Sunitinib only | MSKCC | 25.5 | 50.9 | 23.6 | 96 | 2 | 28 | 65 | 15 | 7.8 | 33.2 | 100 | N/R |
| Motzer et al. [46] (2014) | Ph II RCT | 233 | Everolimus followed by sunitinib versus sunitinib followed by everolimus | MSKCC | 30 | 56 | 14 | 85 | 1 | 25 | 52 | 14 | 10.7 | 32.0 | N/R | N/R |
| Schnadig et al. [27] (2014) | Observational | 134 | Sunitinib only | MSKCC (IMDC) | 11.2 (4.5) | 28.4 (56.7) | 8.2 (23.1) | 82.8 | 2.2 | 14.2 | 18.7 | N/R | 7.5 | 15.5 | 85.1 | N/R |
| Eichelberg et al. [17] (2015) | Ph III RCT | 183 | Sorafenib followed by sunitinib versus sunitinib followed by sorafenib | MSKCC | 45 | 51 | 0.5 | 84 | 3.4 | 26 | 35 | N/R | 8.5 | 30.2 | N/R | 58.5 |
| Gore et al. [30] (2015) | Expanded access trial | 4,543 | Sunitinib only | MSKCC | 20 | 33 | 26 | 88 | 1 | 14 | 45 | 19 | 9.4 | 18.7 | 95 | N/R |
| Pan et al. [47] (2015) | Observational | 50 | Sunitinib only | MSKCC | 20 | 56 | 24 | 84.6 | 4 | 10 | 28 | 58 | 9.4 | N/R | N/R | N/R |
| Tan et al. [29] (2015) | Observational | 1114 | Sunitinib only | MSKCC (IMDC) | 11.3 (13.4) | 46.4 (51.3) | 42.3 (35.3) | 82.7 | 0 | 31.44 | 34.34 | 34.34 | 7.94 | 21.84 | N/R | 58.6 |
| Ruiz-Morales et al. [48] (2016) | Observational | 6,519 | Sunitinib versus pazopanib | IMDC | 23 | 57 | 20 | 90 | N/R | N/R | N/R | N/R | 8.4 | 22.3 | N/R | N/R |
| Rini et al. [49] (2016) | Ph III RCT | 135 | Sunitinib versus pembrolizumab plus axitinib | IMDC | 26 | 71 | 3 | 100 | N/R | N/R | N/R | N/R | 15.1 | 6 | 87 | 41 |
| Choueiri et al. [18] (2017) | Ph II RCT | 78 | Sunitinib versus cabozantinib | IMDC | 0 | 80.9 | 19.1 | 100 | 0 | 11.5 | 42.3 | 25.6 | 5.6 | 21.8 | 98.6 | 68.1 |
| Noize et al. [22] (2017) | Observational | 302 | Sunitinib only | N/R | N/R | N/R | N/R | 83.1 | 2.3 | 28.8 | 40.1 | 18.5 | 8.4 | 23.6 | 97 | 58.3 |
| Lalani et al. [50] (2017) | Observational | 577 | Sunitinib or pazopanib | IMDC | 22 | 58 | 20 | 83.7 | N/R | N/R | N/R | N/R | 11.0 | 31.7 | N/R | N/R |
| Zhang et al. [51] (2017) | Observational | 362 | Sunitinib versus sorafenib | MSKCC (IMDC) | 41.7 (39.8) | 53.0 (51.7) | 5.2 (8.6) | 91.4 | 1.1 | 19.9 | 67.4 | 11.6 | 10.0 | 24.0 | N/R | N/R |
| Motzer et al. [52] (2018) | Ph III RCT | 546 | Nivolumab plus ipilimumab versus sunitinib | IMDC | 23 | 61 | 16 | 100 | 1 | 25 | 45 | 17 | 8.4 | 26 | 97 | 63 |
| Poprach et al. [53] (2019) | Observational | 1,0166 | Multiple TKIs | MSKCC | 27.8 | 64.7 | 7.6 | 95.3 | 5.1 | 23.0 | 38.2 | 22.2 | 10.8 | 31.9 | 30.1 | 7.7 |
| Choueiri et al. [54] (2019) | Ph III RCT | 328 | Nivolumab plus cabozantinib versus sunitinib | IMDC | 22 | 57.3 | 20.7 | 100 | 4.6 | 22.6 | 42.1 | 13.7 | 8.3 | 6 | 99.1 | 70.6 |
| Motzer et al. [28] (2019) | Ph III RCT | 444 | Sunitinib versus avelumab plus axitinib | MSKCC (IMDC) | 22.5 (21.6) | 66.0 (62.2) | 10.1 (16.0) | 100 | 1.8 | 23.9 | 45.5 | 18.7 | 8.4 | 37.87 | 99.3 | 71.5 |
| Rini et al. [55] (2019) | Ph III RCT | 429 | Pembrolizumab plus axitinib versus sunitinib | IMDC | 30.5 | 57.3 | 12.1 | 100 | 1.9 | 33.8 | 39.4 | 17.0 | 11.1 | 6 | 99.5 | 70.6 |
| Savard et al. [23] (2020) | Retrospective database study | 1,769 | Sunitinib only | IMDC | 18 | 58 | 24 | 100 | 3.7 | 28.7 | 38.2 | 29.4 | 8.1 | 28.6 | N/R | N/R |
| Schmidinger et al. [26] (2020) | Observational | 467 | Sunitinib only | MSKCC (IMDC) | 16.6 (15.7) | 29.1 (25.2) | 7.5 (10.3) | N/R | N/R | N/R | N/R | N/R | 10.4 | 34.0 | N/R | N/R |
| Choueiri et al. [56] (2021) | Ph III RCT | 328 | Sunitinib versus nivolumab plus cabozantinib | IMDC | 22.0 | 57.3 | 20.7 | 100 | 4.6 | 22.6 | 42.1 | 13.7 | 8.3 | 34.38 | 99.1 | 70.6 |
| Costello et al. [57] (2021) | Observational | 75 | Sunitinib or pazopanib | IMDC | 14.7 | 65.3 | 20.0 | 78,5 | N/R | N/R | N/R | N/R | 5.69 | 26.2 | N/R | N/R |
| Motzer et al. [58] (2021) | Ph III RCT | 357 | Sunitinib versus lenvatinib plus pembrolizumab versus lenvatinib plus everolimus | IMDC | 27.2 | 63.9 | 9 | 100 | 4.2 | 31.9 | 38.1 | 14.0 | 9.2 | 6 | 98.5 | 71.8 |
| Escudier et al. [59] (2022) | Observational | 3,172 | Sunitinib or pazopanib or others | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | 21 | N/R | N/R |
| Nikic et al. [25] (2022) | Observational | 673 | Sunitinib or pazopanib | MSKCC | 32.6 | 67.4 | N/R | 100 | 3.4 | 21.7 | 51.8 | 8.9 | 14 | 17 | N/R | 27.3 |
| van de Laar et al. [24] (2022) | Observational | 34 | Sunitinib or pazopanib or nivolumab plus ipilimumab or others | IMDC | 9 | 38 | 53 | 56 | N/R | N/R | N/R | N/R | 6.9 | 28.1 | N/R | N/R |
| Author, publication year . | Study type . | Pts receiving sunitinib, n . | Study drugs/comparisons . | Risk score used . | Risk stratification, % . | Percentage of ccRCC pts, % . | ORR, % . | PFS [months], median . | OS [months], median . | AE rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fav. . | int. . | poor . | CR . | PR . | SD . | PD . | all, % . | grade 3/4, % . | ||||||||
| STAR-TOR: this data set | Observational | 702 | Sunitinib or axitinib or temsirolimus | MSKCC | 12.6 | 62.2 | 25.2 | 81.6 | 6.0 | 24.8 | 33.0 | 21.8 | 6.7 | 25.0 | 66.3 | 13.9 |
| Motzer et al. [15] (2009) | Ph III RCT | 375 | Sunitinib versus IFN-α | MSKCC | 38 | 56 | 6 | 100 | 3 | 44 | 40 | 7 | 11.0 | 26.4 | N/R1 | N/R |
| Motzer et al. [45] (2012) | Ph II RCT | 1462 | Sunitinib only | MSKCC | 25 | 60 | 15 | 100 | 0 | 32 | 43 | N/R | 8.5 | 23.1 | N/R | N/R |
| Motzer et al. [16] (2013) | Ph III RCT | 548 | Sunitinib versus pazopanib | MSKCC (IMDC) | 27 (25) | 59 (26) | 9 (17) | 100 | <1 | 24 | 44 | 19 | 9.5 | 29.3 | 99 | 57/173 |
| Miyake et al. [21] (2014) | Observational | 102 | Sunitinib only | MSKCC | 25.5 | 50.9 | 23.6 | 96 | 2 | 28 | 65 | 15 | 7.8 | 33.2 | 100 | N/R |
| Motzer et al. [46] (2014) | Ph II RCT | 233 | Everolimus followed by sunitinib versus sunitinib followed by everolimus | MSKCC | 30 | 56 | 14 | 85 | 1 | 25 | 52 | 14 | 10.7 | 32.0 | N/R | N/R |
| Schnadig et al. [27] (2014) | Observational | 134 | Sunitinib only | MSKCC (IMDC) | 11.2 (4.5) | 28.4 (56.7) | 8.2 (23.1) | 82.8 | 2.2 | 14.2 | 18.7 | N/R | 7.5 | 15.5 | 85.1 | N/R |
| Eichelberg et al. [17] (2015) | Ph III RCT | 183 | Sorafenib followed by sunitinib versus sunitinib followed by sorafenib | MSKCC | 45 | 51 | 0.5 | 84 | 3.4 | 26 | 35 | N/R | 8.5 | 30.2 | N/R | 58.5 |
| Gore et al. [30] (2015) | Expanded access trial | 4,543 | Sunitinib only | MSKCC | 20 | 33 | 26 | 88 | 1 | 14 | 45 | 19 | 9.4 | 18.7 | 95 | N/R |
| Pan et al. [47] (2015) | Observational | 50 | Sunitinib only | MSKCC | 20 | 56 | 24 | 84.6 | 4 | 10 | 28 | 58 | 9.4 | N/R | N/R | N/R |
| Tan et al. [29] (2015) | Observational | 1114 | Sunitinib only | MSKCC (IMDC) | 11.3 (13.4) | 46.4 (51.3) | 42.3 (35.3) | 82.7 | 0 | 31.44 | 34.34 | 34.34 | 7.94 | 21.84 | N/R | 58.6 |
| Ruiz-Morales et al. [48] (2016) | Observational | 6,519 | Sunitinib versus pazopanib | IMDC | 23 | 57 | 20 | 90 | N/R | N/R | N/R | N/R | 8.4 | 22.3 | N/R | N/R |
| Rini et al. [49] (2016) | Ph III RCT | 135 | Sunitinib versus pembrolizumab plus axitinib | IMDC | 26 | 71 | 3 | 100 | N/R | N/R | N/R | N/R | 15.1 | 6 | 87 | 41 |
| Choueiri et al. [18] (2017) | Ph II RCT | 78 | Sunitinib versus cabozantinib | IMDC | 0 | 80.9 | 19.1 | 100 | 0 | 11.5 | 42.3 | 25.6 | 5.6 | 21.8 | 98.6 | 68.1 |
| Noize et al. [22] (2017) | Observational | 302 | Sunitinib only | N/R | N/R | N/R | N/R | 83.1 | 2.3 | 28.8 | 40.1 | 18.5 | 8.4 | 23.6 | 97 | 58.3 |
| Lalani et al. [50] (2017) | Observational | 577 | Sunitinib or pazopanib | IMDC | 22 | 58 | 20 | 83.7 | N/R | N/R | N/R | N/R | 11.0 | 31.7 | N/R | N/R |
| Zhang et al. [51] (2017) | Observational | 362 | Sunitinib versus sorafenib | MSKCC (IMDC) | 41.7 (39.8) | 53.0 (51.7) | 5.2 (8.6) | 91.4 | 1.1 | 19.9 | 67.4 | 11.6 | 10.0 | 24.0 | N/R | N/R |
| Motzer et al. [52] (2018) | Ph III RCT | 546 | Nivolumab plus ipilimumab versus sunitinib | IMDC | 23 | 61 | 16 | 100 | 1 | 25 | 45 | 17 | 8.4 | 26 | 97 | 63 |
| Poprach et al. [53] (2019) | Observational | 1,0166 | Multiple TKIs | MSKCC | 27.8 | 64.7 | 7.6 | 95.3 | 5.1 | 23.0 | 38.2 | 22.2 | 10.8 | 31.9 | 30.1 | 7.7 |
| Choueiri et al. [54] (2019) | Ph III RCT | 328 | Nivolumab plus cabozantinib versus sunitinib | IMDC | 22 | 57.3 | 20.7 | 100 | 4.6 | 22.6 | 42.1 | 13.7 | 8.3 | 6 | 99.1 | 70.6 |
| Motzer et al. [28] (2019) | Ph III RCT | 444 | Sunitinib versus avelumab plus axitinib | MSKCC (IMDC) | 22.5 (21.6) | 66.0 (62.2) | 10.1 (16.0) | 100 | 1.8 | 23.9 | 45.5 | 18.7 | 8.4 | 37.87 | 99.3 | 71.5 |
| Rini et al. [55] (2019) | Ph III RCT | 429 | Pembrolizumab plus axitinib versus sunitinib | IMDC | 30.5 | 57.3 | 12.1 | 100 | 1.9 | 33.8 | 39.4 | 17.0 | 11.1 | 6 | 99.5 | 70.6 |
| Savard et al. [23] (2020) | Retrospective database study | 1,769 | Sunitinib only | IMDC | 18 | 58 | 24 | 100 | 3.7 | 28.7 | 38.2 | 29.4 | 8.1 | 28.6 | N/R | N/R |
| Schmidinger et al. [26] (2020) | Observational | 467 | Sunitinib only | MSKCC (IMDC) | 16.6 (15.7) | 29.1 (25.2) | 7.5 (10.3) | N/R | N/R | N/R | N/R | N/R | 10.4 | 34.0 | N/R | N/R |
| Choueiri et al. [56] (2021) | Ph III RCT | 328 | Sunitinib versus nivolumab plus cabozantinib | IMDC | 22.0 | 57.3 | 20.7 | 100 | 4.6 | 22.6 | 42.1 | 13.7 | 8.3 | 34.38 | 99.1 | 70.6 |
| Costello et al. [57] (2021) | Observational | 75 | Sunitinib or pazopanib | IMDC | 14.7 | 65.3 | 20.0 | 78,5 | N/R | N/R | N/R | N/R | 5.69 | 26.2 | N/R | N/R |
| Motzer et al. [58] (2021) | Ph III RCT | 357 | Sunitinib versus lenvatinib plus pembrolizumab versus lenvatinib plus everolimus | IMDC | 27.2 | 63.9 | 9 | 100 | 4.2 | 31.9 | 38.1 | 14.0 | 9.2 | 6 | 98.5 | 71.8 |
| Escudier et al. [59] (2022) | Observational | 3,172 | Sunitinib or pazopanib or others | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | 21 | N/R | N/R |
| Nikic et al. [25] (2022) | Observational | 673 | Sunitinib or pazopanib | MSKCC | 32.6 | 67.4 | N/R | 100 | 3.4 | 21.7 | 51.8 | 8.9 | 14 | 17 | N/R | 27.3 |
| van de Laar et al. [24] (2022) | Observational | 34 | Sunitinib or pazopanib or nivolumab plus ipilimumab or others | IMDC | 9 | 38 | 53 | 56 | N/R | N/R | N/R | N/R | 6.9 | 28.1 | N/R | N/R |
AE, adverse event; ccRCC, clear cell renal cell carcinoma; CR, complete remission; N/R, not reported; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression free survival; PR, partial remission; RCT, randomized controlled trial; SD, stable disease.
1Not reported.
2Schedule 4/2 patients.
3grade 3 and grade 4, respectively.
437.5 mg/d sunitinib for 4 weeks, then 2 weeks off, only first-line pts.
5Not reached.
6Only pts <70 years.
7Haanen et al., J Clin Oncol 39, no.15_suppl (May 20, 2021) 4574.
8Powles et al., J Clin Oncol 40, no. 6_suppl (Feb 20, 2022) 350.
9Time to treatment discontinuation.
Survival Endpoints
Comparing the STAR-TOR real-world data to the first phase III trial published by Motzer et al. [3, 15] in 2007 and 2009, it strives the reader’s attention that the clinical efficacy is inferior with a PFS of 6.7 months (OS 25 months) in the STAR-TOR registry compared to 11 months (OS 26.4 months) published by Motzer et al. [3, 15]. A similar, albeit not as pronounced trend can be observed for almost all subsequent sunitinib trials: PFS usually ranged from 8 to 11 months. We performed an explorative analysis according to histology which may explain the detrimental effect of non-clear cell histologies for this finding: clear cell a/mRCC patients yielded a PFS of 7.4 months compared to 5.0 months for all other histologies. These 7.4 months (7.7 months for 477 first-line ccRCCs) do not markedly differ from the results of other published real-world data [21‒24]. Only Nikic et al. [25] and Schmidinger et al. [26] reported relevantly larger PFS of 10.4 and 14 months. For OS, time ranged from 15.5 to up to 37.8 months [27, 28]. Here, our analyses yielded a survival of 25 months that might be situated close to the mean. According to our subgroup analysis, clear cell a/mRCC patients had a median OS of 25.7 months compared to non-ccRCC patients with 20.9 months. Furthermore, the reason for the short PFS in our study might be due to the large proportion of patients with poor risk of disease which was 25% compared to 6% in the studies by Motzer et al. [3, 15]. Similarly, most of the other studies derived their data from participants with better prognosis scores. Yet, studies with proportions of poor-risk patients comparable to the STAR-TOR data (around 25%) showed comparable OS and PFS [21, 23, 24, 29, 30].
It is widely accepted and seems to be common knowledge in oncology that the therapeutic efficacy of antineoplastic agents decreases with treatment line. This was exemplarily shown in the SWITCH trial which compared the sequential treatment of a/mRCC patients with sunitinib followed by sorafenib and vice versa [17]. While sunitinib in this trial in first line yielded a median PFS of 8.5 months, the PFS deteriorated to 5.4 months when used in second-line after first-line sorafenib. For sorafenib, this effect was even worse. Another example for this widely known effect was the GERCOR study published by Tournigand et al. [31] in 2004 for the sequential treatment in colorectal cancer. Therefore, we found it even more surprising that we could not observe this phenomenon in our data set. As is shown in Table 3 and was outlined in the Results part of our publication, we observed no relevant deterioration of clinical efficacy of sunitinib until the fourth treatment line.
Subgroup Analyses
Our analyses showed that the survival results are very different according to histologic subtype with ccRCC reaching a numerically longer survival compared to non-ccRCC. This corresponds to findings of the meta-analysis by Vera-Badillo et al. [32]. Similarly, Noize et al. [22] showed better survival for ccRCC patients upon treatment with sunitinib. A prospective trial published by Tannir et al. [33] in 2012 showed a median PFS of 2.7 months in a mixed group of non-clear cell a/mRCC patients, whereas Armstrong et al. [34] showed in the ASPEN trial a median PFS of 8.3 months (OS 31.5 months) for sunitinib-treated patients. Later, Tannir et al. [35] detected in 2016 a median PFS of 6.1 months (OS not reached at the time of publication) for sunitinib in the ESPN trial. Obviously, the treatment effect in non-clear cell a/mRCC depends very much on the variety of treated histological subtypes. The STAR-TOR non-clear cell cohort with predominantly papillary histology yielded a median PFS of 5.0 months and a median OS of 20.9 months compared relatively well in this context.
With respect to BMI, our data confirm low BMI (<25) to be a negative predictor for OS and PFS. This has been shown previously for TKI treatment by several studies [36‒39]. In correspondence to these observations, it has been shown that patients with BMI <25 seem to experience more dose-limiting toxicities [40].
Similarly, MSKCC risk score as a well-established predictor of survival has been clearly confirmed by our data [21, 41]. Our study confirmed patients’ serum LDH levels were identified as a prognostic marker for OS and PFS with lower LDH levels being associated with better survival. This has been described by Motzer et al. [42] for sunitinib. In addition, high serum LDH levels are well known to be associated with poor survival in other solid tumor entities [43].
Lastly, in our study population, patients with different nephrectomy statuses had different survival times: whereas median PFS and OS were very similar for patients with nephrectomy before the diagnosis of metastatic disease and patients with already known metastatic disease at the time point of surgery, shorter survival times were detected for patients without nephrectomy. This effect seemed to be at least partially driven by the MSKCC risk score, with a higher proportion of poor-risk MSKCC patients not receiving nephrectomy. As our findings are based on retrospective cohort study data, the intention of the nephrectomy such as a “cytoreductive” surgery is impossible to determine. Comparisons to studies specifically evaluating cytoreductive nephrectomy should be made with caution, but we believe that our results reinforce the claim for further evidence [44].
Adverse Events
Regarding sunitinib-related AEs, the real-world registry character of our study must be taken into account. On one hand, the event rates (in total 66.1%, grade 3/4 13.8%) of the three predominant types of AEs (gastrointestinal disorders, general disorders, and skin and subcutaneous tissue disorders) are also high in other real-world data sets: Miyake et al. [21] reported even 100% AEs of all grades with similar symptoms but mentioning hypothyroidism and hypertension as being equally frequent. Grade 3/4 AEs were reported as follows: thrombocytopenia 53.6%, leucopenia 20.9%, fatigue 20%, hand-foot syndrome 13.6%, and arterial hypertension 10.9%.
Yet, the STAR-TOR data seem to underestimate grade 3/4 AEs: Nikic et al. [25] report grade 3/4 AEs in 27.3% of the patients, whereas Noize et al. [22] described grade 3/4 AEs in 58.3% of the patients. Even these rates of real-world data are often much lower than AE rates reported by randomized controlled trials. As shown by Table 5, the rates of grade 3/4 AEs usually range between almost 60 and around 70%. The reason for these discrepancies might be the high prevalence of already pretreated hypertension in a real-world setting, masking sunitinib side effects.
Limitations
Several limitations must be considered when interpreting our results. First, several variables suffer from missing data. Particularly, MSKCC score was not available in 30.2% of the patients and therefore may have hampered our statistical testing. Missing data are a common issue in real-world studies: Schmidinger et al. [26] similarly report missingness in 49% of the patients. Van Laar reported missing performance status in 41% of the patients [24]. Several here-discussed real-world studies did not even transparently report missing data [21, 23, 25]. Nevertheless, it must be repeated that missingness at random cannot be assumed and that some of the results might be distorted by data unavailability. However, the monitoring of participating STAR-TOR centers may have reduced the clinically relevant bias.
Second, our study and many phase IV observational studies are prone to some extent of reporting bias. Even though all STAR-TOR study sites were supposed to use RECIST 1.1. criteria, some of the outcome measures may lack precision. Similarly, some physicians might have omitted using CTCAE for documentation of AEs. Also, in both cases the random monitoring visits of the study centers should have diminished the extent of bias.
Nevertheless, the STAR-TOR data on sunitinib treatment must be considered as an important step toward complete understanding for treatment patterns and response in clinical routine. The study size together with the prospective data collection and the possibility of subgroup analyses are unique. Its study population with a high proportion of patients with unfavorable MSKCC poor-risk score paves the way for comparisons yet to be published real-world data from the ICI combination era. It therefore will facilitate the development of clinical decision models evaluating the patients’ fitness for ICI therapy.
Conclusions
This prospective real-world data study adds further evidence to the persisting relevance of sunitinib in treatment of patients with a/mRCC who cannot receive or tolerate ICIs. The study population includes a high proportion of patients with unfavorable MSKCC poor-risk score, but shows still reasonably good PFS and OS results, while the drug demonstrates a favorable safety profile. Subgroup analyses confirmed worse prognosis score according to MSKCC, low BMI, and high LDH levels to be negative predictive markers for sunitinib effectiveness, what should be considered for clinical decision models.
Statement of Ethics
The STAR-TOR registry received prior approval by the Ethics Committee of Münster University Hospital in 2007 (Nr.2007-484-f-S) and was registered in the US library of medicine database (NCT00700258). The study received prior ethical approval (Ethics Committee of Münster University Hospital in 2007 [Nr.2007-484-f-S]). Written informed consent was obtained from all participants (or their parent/legal guardian/next of kin) to participate in the study. All the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Conflict of Interest Statement
Michael Woike is an employee of Pfizer Germany. Annemarie Uhlig has received honoraria, travel, and accommodations by Pfizer and APOGEPHA. Katrin Schlack received fees as speaker, consultant, or advisor and travel expenses from APOGEPHA, BMS, Eisai, EUSA Pharma, Ipsen, Merck Healthcare, MSD, Novartis, and Pfizer. Johannes Uhlig, Thomas Fischer, Lutz Trojan, Lothar Bergmann, Martin Bögemann, Peter J. Goebell, Michael Rink, Mathias Reichert, Marianne Leitsmann, and Arne Strauß have no conflicts of interest to disclose.
Funding Sources
The STAR-TOR registry was funded by Pfizer Pharma GmbH, Berlin, Germany.
Author Contributions
Conceptualization of the research project: A.U., J.U., M.W., M.L., and A.S. Design of the methodology and contribution to data analysis: A.U., J.U., M.W., and T.F. Data collection: L.T., L.B., M.B., P.J.G., M.Ri., M.Re., K.S., M.L., and A.S. Data curation: M.W. and T.F. Visualization: T.F. and A.U. Writing of the manuscript: A.U., J.U., M.W., M.L., M.Ri., and M.Re. Reviewing and editing: T.F., L.T., L.B., M.B., P.J.G., M.R., K.S., M.Ri., and M.Re.
Data Availability Statement
The data that support the findings of this study are not publicly available for company data restriction regulations. Pfizer is the owner of these data and does not disclose patient-related information. Requests regarding the availability of data that support the findings of this study may be directed to Pfizer Pharma GmbH, Friedrichstrasse 110, 10117 Berlin, Germany.