Abstract
Introduction: We report a rare case of Skene’s gland hyperplasia where the serum prostate-specific antigen (PSA) level was measurable. Case Presentation: The patient was a 91-year-old woman with a suspected bladder mass at the bladder trigone. Cystoscopy revealed a suspected lesion and an obstructed anterior bladder neck with a large mass located from a “7 o’clock” to “11 o’clock” area. The photodynamic diagnosis was negative. Transurethral subtotal resection of the mass was performed. The serum PSA level at the third postoperative day was 0.08 ng/mL. Postoperative cystography showed no contrast media extravasation. Thus, histopathology revealed massive adenomyomatous hyperplasia of the Skene’s gland, as well as nondysplastic urothelium and glandular and squamous epithelium. Immunohistochemistry showed strong PSA and NKX3.1 positivity, confirming the diagnosis of “female prostate.” FISH analysis showed only green signals that confirm an XX karyotype. In follow-up to 17 months, there was no disease recurrence or need for a urinary catheter. Conclusion: Effective therapeutic strategies for these lesions are unknown due to the absence of reported cases. Given the patient’s age, we assumed that bladder neck resection by transurethral resection with a controlled level of serum PSA would be a suitable therapeutic approach.
Introduction
The female prostate (Skene’s gland) develops from epithelial buds located inside the urogenital sinus [1]. The Skene’s paraurethral glands are situated on the anterior wall of the vagina, around the clitoris, and the external urethral orifice. Histologically, they are composed of glands, ducts, and connective musculofibrous tissues, with the opening of ducts into the lumen of the urethra [2]. The morphophysiological, histochemical-enzymatic, and immunohistochemical aspects of the female prostate are similar to the male prostate [3]. The prostate (Skene’s gland) in females is the primary source of the prostatic specific antigen (PSA), just as it is in males [4]. Here, we report a case of female prostate hyperplasia at the bladder neck, where the serum PSA level was measurable.
Case Report
Clinical Presentation
A 91-year-old woman presented at our department for a suspected bladder leiomyoma of unspecified duration. The patient had an indwelling urinary catheter due to acute urinary retention with bladder neck obstruction by the suspected mass. Her medical history included urosepsis, cervical polyps, uterine prolapse, low-T3 syndrome, and atrial fibrillation.
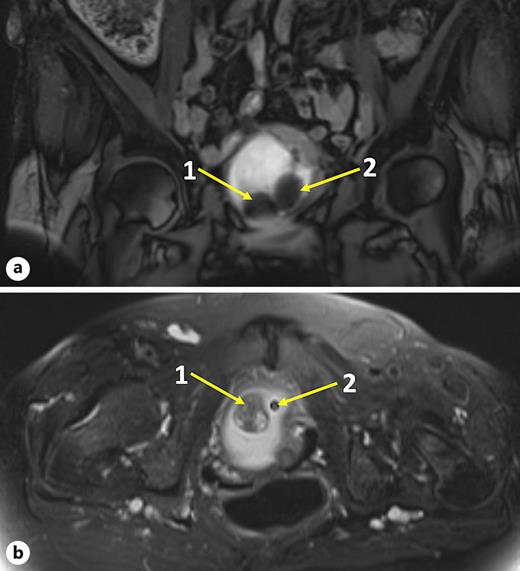
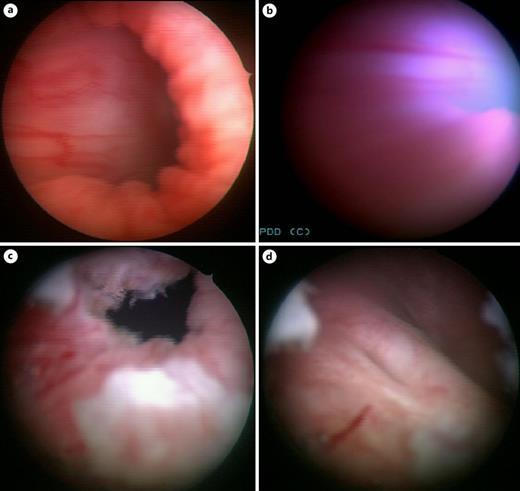
Physical examination revealed no flank masses. Abdominal ultrasound raised the suspicion of a bladder lesion. Urinalysis revealed no white cells or bacteria in the stained urinary sediment. A magnetic resonance imaging of the pelvis and abdomen revealed a sessile bladder mass (2.8 cm) at the bladder trigone on the right side, without perivesical infiltration or regional lymphadenopathy described as leiomyoma, as shown in Figure 1. Cystoscopy revealed the suspected bladder leiomyoma and an obstructed anterior bladder neck with a large mass located from a “7 o’clock” to “11 o’clock” area. Typical redness of the tissue was detected because of the indwelling urinary catheter. The photodynamic diagnosis was negative, as shown in Figure 2b.
MRI of the pelvis. a T1. b T2 sagittal section. In both panels, “1” indicates the 2.8 × 2.4 × 2.0 cm suspected lesion and “2” indicates the urinary catheter.
MRI of the pelvis. a T1. b T2 sagittal section. In both panels, “1” indicates the 2.8 × 2.4 × 2.0 cm suspected lesion and “2” indicates the urinary catheter.
Cystoscopic identification of the lesion. a Obstructed bladder neck (preresection). b Photodynamic diagnosis. c Results after surgical resection. d Postoperative view with the anatomical ureteral opening on both sides.
Cystoscopic identification of the lesion. a Obstructed bladder neck (preresection). b Photodynamic diagnosis. c Results after surgical resection. d Postoperative view with the anatomical ureteral opening on both sides.
Treatment and Follow-Up
Transurethral resection of the mass to the level of the detrusor muscle was performed. Simultaneously, two endocervical polyps were identified and biopsies were performed. Because of the tumor’s location, a cystography was performed on the third postoperative day. The examination showed no contrast media extravasation, but vesicoureteral reflux on the right side was observed. The serum PSA at the third postoperative day (timepoint of diagnosis of the prostatic tissue level) was 0.08 ng/mL. The patient was discharged after 5 days of hospital stay. In follow-up to 17 months, there was no disease recurrence and no need for a urinary catheter.
Histopathology
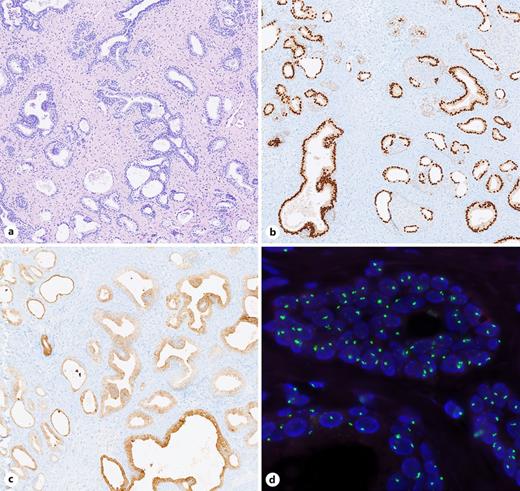
The 14.5 × 2.5 × 0.5 cm section of the resected specimen revealed pinkish-gray tissue. Microscopic evaluation revealed urothelium and glandular and squamous epithelium without any dysplasia. In addition, massive adenomyomatous hyperplasia of the Skene’s gland was detected. Immunohistochemical staining showed strong PSA and NKX3.1 positivity, and the female karyotype was confirmed by fluorescent in situ hybridization to rule out contamination by male prostatic tissues, as shown in Figure 3. Moreover, polyp biopsy expressed nondysplastic leukoplakias, acanthosis, and the enlargement of the stratified squamous epithelium with minimal persistent inflammation.
Histopathology. Scale bar (a-c): 500 μm. a H&E staining showing a glandular architecture and fibrous stroma with a prostate-like configuration. b Epithelial cells are strong nuclear positive for NKX3.1. c PSA staining displays a cytoplasmic positivity in the epithelial cells. d In the FISH analysis, two green signals (X chromosome) are visible with no orange signals (Y chromosome), confirming an XX karyotype.
Histopathology. Scale bar (a-c): 500 μm. a H&E staining showing a glandular architecture and fibrous stroma with a prostate-like configuration. b Epithelial cells are strong nuclear positive for NKX3.1. c PSA staining displays a cytoplasmic positivity in the epithelial cells. d In the FISH analysis, two green signals (X chromosome) are visible with no orange signals (Y chromosome), confirming an XX karyotype.
Discussion
Immunohistochemical studies have shown that androgenic receptors are also present in the human female prostate, which highlights the role of androgens in maintaining the prostate’s structure and function in the female body [1, 5]. Additionally, extended androgenic stimulation may play a role in developing ectopic prostatic tissues [1]. Custadio et al. [3] have shown in their experiment in female gerbils (Meriones unguiculatus) that alterations that affect this gland during the aging process are prostatic hyperplasia, microcalculi, prostate calculi, prostatitis, and adenocarcinoma. The 15 cases of adenocarcinomas of the paraurethral Skene glands, identified in elderly female patients, presented distinct similarities to analogous lesions in the male prostate, and 8 cases were associated with increased PSA levels (range: 0.8–60.8 ng/mL) [6].
The presence of ectopic prostatic tissue in the female genitourinary tract is extremely rare [5]. It has occasionally been described in the uterine cervix, vagina, and ovaries, including benign and malignant patterns, as shown in Table 1. Biancardi et al. [7] have demonstrated that abnormal prenatal exposure to testosterone in female gerbils facilitates the development of ectopic prostatic tissue associated with adenomatous hyperplasia. To our awareness, this is the second documented occurrence of an adenomyomatous hyperplasia of paraurethral Skene glands, as shown in Table 2.
Ectopic prostatic tissue in the human female
| Source . | Ages, years . | Anatomic site . | PSA . | Malignancy . |
|---|---|---|---|---|
| Smith et al. [8] | 70 | Ovary | Positive | No |
| McCluggate et al. [9] | 21, 23, 25, 32, 61 | Cervix | 3 of 6 positive | None |
| 65 | Vagina | |||
| Kelly et al. [10] | 43, 54 | Vulva | Positive | None |
| Halat et al. [5] | 75 | Urethra | Not reported | No |
Comparison of the clinical and morphological features of the two known published cases of Skene’s gland hyperplasia
| Source . | Age, years . | Anatomic site . | Size, cm . | PSA . | NKX3.1 . | PrAP . | FISH analysis . | PSA antigen, ng/mL . |
|---|---|---|---|---|---|---|---|---|
| Kazakov et al. [11] | 59 | Posterior urethra | 4.5 | Positive | Not reported | Positive | Not reported | Not reported |
| Current case | 91 | Bladder neck | 2.8 | Positive | Positive | Not reported | XX karyotype | 0.08 |
| Source . | Age, years . | Anatomic site . | Size, cm . | PSA . | NKX3.1 . | PrAP . | FISH analysis . | PSA antigen, ng/mL . |
|---|---|---|---|---|---|---|---|---|
| Kazakov et al. [11] | 59 | Posterior urethra | 4.5 | Positive | Not reported | Positive | Not reported | Not reported |
| Current case | 91 | Bladder neck | 2.8 | Positive | Positive | Not reported | XX karyotype | 0.08 |
PSA, prostate-specific antigen; PrAP, prostatic acid phosphatase; FISH, fluorescent in situ hybridization.
However, we reported for the first time a measurable serum PSA level of 0.08 ng/mL (lower limit <0.04 ng/mL) in a human female patient with benign prostatic hyperplasia tissue and strong PSA and NKX3.1 positivity. In the previously reported case, the authors found a proliferation of glands and myomatous stromal nodules. Although the glandular component was immunoreactive to PSA and prostatic acid phosphatase, the basal cell hyperplasia specimen was immunonegative [11].
In the present case, we performed a subtotal resection, considering the patient’s age. However, in other patients, total resection may be considered through minimally invasive or open surgery, depending on the patient’s age and the lesion’s location.
In summary, Skene’s gland hyperplasia in women is extremely rare. To our awareness, there were no previous reports on appropriate therapy. Serum PSA level can be a reliable indicator for confirming the presence of the lesion when breast cancer or polycystic ovary syndrome has been excluded. Fluorescent in situ hybridization analysis is required to establish an XX karyotype. In such cases, surgical resection by transurethral resection with controlled serum PSA level may be an appropriate therapy.
Acknowledgments
We would like to thank Michael Hanna, PhD (Mercury Medical Research and Writing), for proofreading the manuscript.
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines. The written informed consent was obtained from the patient for publication of the details of the medical case and accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources received.
Author Contributions
Data collection and manuscript writing: R.G.; performance of the operation: B.A.; histology description: S.S. and S.M.; data analysis and interpretation: R.G., B.A., A.S., S.S., J.B., and S.M.; and manuscript editing: B.A., J.B., A.S., S.S., and S.M.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.