Abstract
Background: The aim of the study was to identify the cooperation of authors, countries, institutions and explore the hot spots regarding research of tyrosine kinase inhibitors (TKIs) for renal cell carcinoma (RCC) treatment in the past 22 years. Summary: Relevant original and review articles were obtained from the Web of Science Core Collection from 2000 to 2022. CiteSpace software was used to perform the visualization of scientific productivity and emerging trends. Network maps were generated to evaluate the collaborations between different authors, countries, institutions, and keywords. Key Messages: A total of 4,951 articles related to TKI for RCC treatment were identified. We observed a gradual increase in the number of publications from 2000 to 2022. The USA dominated the field in all countries, and Mem Sloan Kettering Cancer Centre (USA) had more extensive cooperating relationships with other institutions. Motzer RJ and Escudier B were two of the authority scholars in this specific field with the most publications and co-citations. Journal of Clinical Oncology had the most citations of all the journals. A total of 10 major clusters were explored based on the reference co-citation analysis. From 2000 to 2022, the research hot spots have undergone two dramatic shifts during 2006 and 2019, respectively, relevant topics were TKI and TKI combined with immune checkpoint inhibitors (CPIs). At present, the research hot spots focus on CPI and targeted therapies. Bibliometric analysis is allowing researchers to recognize the current research status by providing a comprehensive overview of the development of scientific literature related to TKI for RCC treatment, and information for further research be demonstrated as well.
Introduction
Renal cell carcinoma (RCC) constitutes the twelfth most common cancer worldwide, accounting for 3% of all adult malignancies, has become a major burden on social economy [1]. It arises from the renal tubular epithelium, which lines in the proximal convoluted tubules and constitutes of tubes in the kidney servicing for transporting urine [2]. In 2012, the International Society of Urological Pathology (ISUP) identified 15 sub-types of RCC that have different genetic and epigenetic characteristics, among these, clear cell RCC (ccRCC) is the most frequent (75–80%) and best-studied sub-type [3]. Around 50% of RCC is detected incidentally, for patients who struggle at an early stage, surgery is confirmed as an effective approach and also been paid adequate attention to [4]. However, approximately 30% of RCC patients unavoidably have metastatic disease at initial diagnosis [5]. The prognosis for patients with intermediate or high-risk RCC after surgical resection has historically been poor, with a 5-year relapse rate of more than 30–40%. Among patients at tumor-node-metastasis stages 1 and 2, 5-year survival rates are only 81% and 74% [6], respectively, especially, falling dramatically to 16% once the disease made metastatic development [7].
Tyrosine kinase inhibitors (TKIs) are the most recommended targeted treatment that stops tumors from being able to grow and spread through the involvement of the von Hippel-Lindau (VHL) gene [8]. Sunitinib as the first-line treatment in metastatic ccRCC is the most widely used TKI. Second-line agents like sorafenib and cabozantinib have demonstrated improvement in disease control and survival while accompanied by manageable safety profiles [9]. All of these agents are characterized by multi-targeted TKI that targets a range of vascular endothelial growth factor receptors in addition to VEGF receptor kinases, including PDGF receptor beta, c-KIT, and FLT3 [10]. Although the agents have made the therapeutic landscape, approximately 10–20% of advanced RCC exhibit primary resistance, and most of the remaining patients initially respond to treatment but develop drug resistance in a short gap [11]. Possible mechanisms point to the activation of alternative receptors, epithelial-to-mesenchymal transition, gene mutations, hypoxia, and hypoxia-inducible factors, modifications of gene expression levels, and the tumor microenvironment [12, 13]. Some studies make further analysis and demonstrate the potential resistance mechanism, like overexpression of mesenchymal markers (vimentin, ZEB1, SLUG, and TWIST protein), Ecadherin, IL6, IL8, and senescence marker SA-β-gal, or lower expression cell migration ability [3, 14].
While TKI dominates the treatment approaches, its resistance has led to new therapy progression. Immune checkpoint inhibitors (CPIs) gradually transformed the oncology treatment landscape in solid tumors, like programmed cell death protein 1 (PD-1) and its ligand (PD-L1) [15]. Drugs combination therapy such as the TKI-CPI combinations initially demonstrates superior outcomes and better patient life quality, providing a potential alternative treatment that is now receiving more attention. Lenvatinib plus pembrolizumab, one of the recommended combination types, indicates a strong association with longer progression-free survival (PFS) and overall survival (OS) than TKI only [16]. Similar inspiring results were observed with lenvatinib plus pembrolizumab and pembrolizumab plus axitinib [17, 18]. Although combinations require us to focus on drug toxicity, the safety and tolerability of these combinations are manageable.
CiteSpace is a web-based Java application for data analysis and visualization [19, 20]. The software adopts multidimensional analysis (countries/regions, institutions, authors, cited author, and cited reference collaboration), cluster analysis, keywords citation burst, timeline view graph, and other methods, providing relevant scientific knowledge maps, analyzing the evolution of numerous research frontiers and development trends in the research field [21]. Much progress has been made in the use of TKI technology for RCC treatment, while there is still no relevant bibliometric visual analysis. In this study, knowledge mapping of TKI for RCC was performed using CiteSpace software to comprehensively investigate collaboration networks and research trends in TKI development.
Data Source and Search Strategy
We accessed the Web of Science core collection database with the following search term: TS = (“carcinoma, renal cell” OR “renal cell carcinomas” OR “nephroid carcinoma” OR “cancer, renal cell” OR “renal adenocarcinoma”) AND (“tyrosine kinase inhibitor” OR “sunitinib” OR “axitinib” OR “pazopanib” OR “sorafenib”). Articles were enrolled if met the following criteria: (1) the time span is between 2000 and 2022; (2) only original articles and reviews written in English about TKIs for RCC were included. Documents excluded from this review include (1) abstracts from meetings and letters, repeated articles, and irrelevant proceedings; (2) non-English articles.
Statistical Analysis
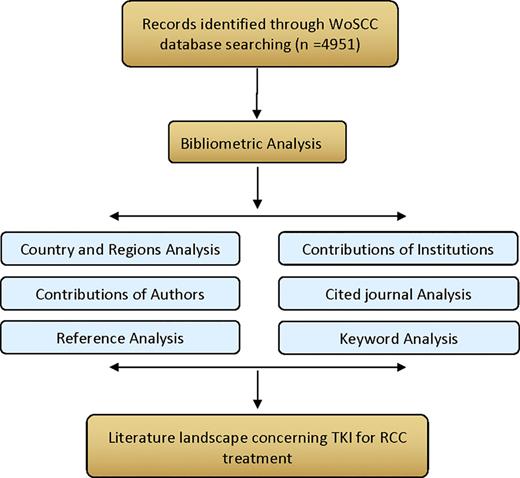
CiteSpace 6.1.R3 was used for bibliometric analysis. The knowledge maps were constructed by types of countries, authors, institutions, and keywords related to TKI for RCC research, which consisted of nodes and links, and the links between nodes represented cooperative/co-occurrence or joint relationships. The color and thickness of the circles in the nodes indicated the number of publications at different epochs. More the publications, the larger is the node. The parameters of CiteSpace were set as follows: time slicing: January 2000 to September 2022, and 2 years were chosen as a time slice. As analytical criteria, the G-index was used. The pruning criteria were pathfinder, pruning of slices, and pruning of merged networks. Cluster degree was measured by using modularity Q (Q) and weighted mean silhouette (S), a clustering structure of Q >0.3 was considered significant, whereas a clustering result of S >0.5 is considered satisfactory. Author, institution, country/region, references, and keywords were chosen for analysis one by one. The detailed procedure of data analysis is shown in Figure 1.
Flowchart of literature selection and scientific bibliometric analysis.
Analysis of Publication Literature Output
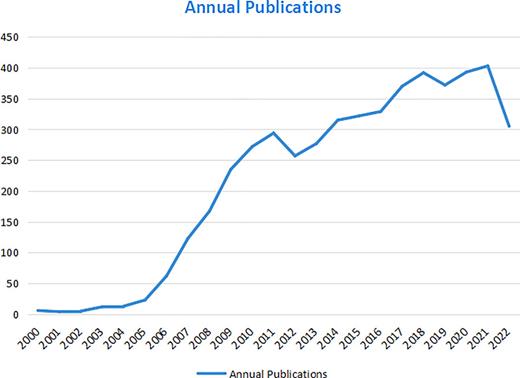
The quantity and growth rate of scientific literature at a given time node can reflect the academic progress in a specific research field and provide essential messages for us to grasp the development potential from a quantitative perspective. From January 1, 2000 to November 1, 2022, a total of 4,951 publications in the Web of Science Core Collection (WoSCC) were extracted, including 3,915 articles and 1,036 reviews. Figure 2 depicts the annual publication output and trend of literature, signifying the growth rate of publications. It could be observed that annual publication output increased annually and has accelerated after 2017. In 2021, 403 articles were published, nearly four times as many as in 2007. There is no hesitation in predicting that the 2022 production will be fully parallel to or no less than the 2021 production. A three-phased history of research can be traced: (i) 2000–2006: the initial stage, in this stage, TKI therapy just entered orbit with less than 100 publications annually; (ii) 2006–2016: rapid development stage, which had a range of 100–350 annual publications, with a constant increase rate; and (iii) 2017–2022: the transformed stage, with CPI gradually entered the research focus.
The output of articles and growth prediction of TKI for RCC from 2000 to 2022.
Analysis of Countries/Regions and Institutions
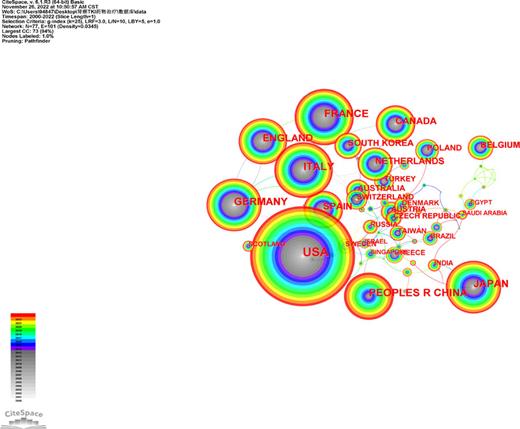
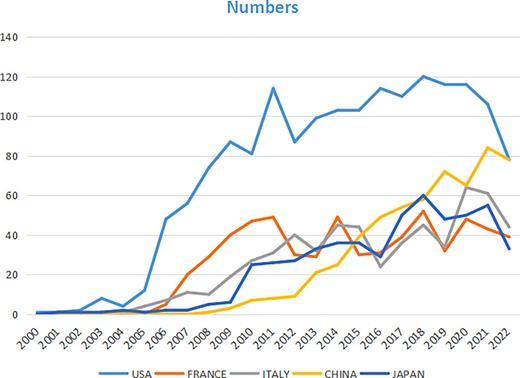
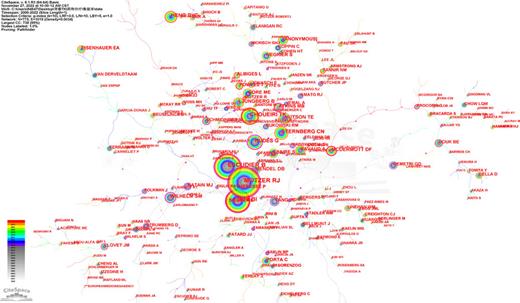
Geographical distribution analysis was taken to evaluate the development level of TKI for RCC in different countries and institutions, more closely, to figure out existent cooperative relationships. A total of 4,951 publications were published by 647 core institutions in 77 central countries or regions. Relevant collaboration networks were noted in Figures 3 and 4. There were 77 nods with 101 links in countries/regions networks (destiny = 0.0345). The top 5 countries are the USA, followed by France, Italy, Peoples R China, and Japan (Table 1). Developed countries including the USA, Germany, and Canada reported the earliest records in 2000. Meanwhile, the data from the USA and Germany illustrated higher centrality than any other members (centrality >0.1), which suggested that the research in these two countries had a higher focus on TKI for RCC. There are four developed countries among the top 5 contribution members. It is clear that in these countries, related research was initiated earlier and received more attention. In detail, Figure 5 demonstrated the number of articles published in each period in the top 5 countries. We could see the researchers’ enthusiasm processed into the fasting track from 2006 in the USA. This may be associated with the fact that TKI was gradually showing a higher effect profile at that time.
The countries/regions collaboration network of TKI for RCC from 2000 to 2022.
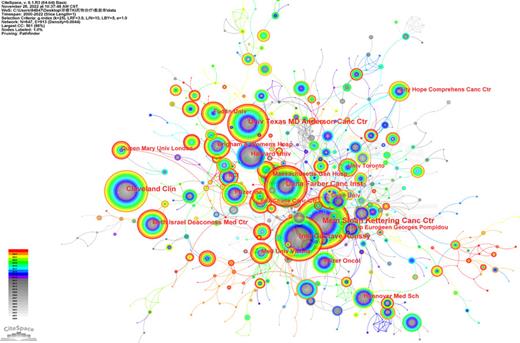
The institutions collaboration network of TKI for RCC from 2000 to 2022.
The top 10 countries and institutions contributed to TKI for RCC
| Rank . | Country . | Centrality . | Count . | Institutes . | Centrality . | Count . |
|---|---|---|---|---|---|---|
| 1 | USA | 0.11 | 1,632 | Mem Sloan Kettering Cancer Centre (US) | 0.13 | 208 |
| 2 | France | 0 | 612 | Inst Gustave Roussy (FR) | 0.03 | 164 |
| 3 | Italy | 0.09 | 581 | Dana Farber Canc Inst (US) | 0.03 | 151 |
| 4 | China | 0 | 573 | Cleveland Clin (US) | 0.02 | 147 |
| 5 | Japan | 0 | 529 | Univ Texas MD Anderson Canc Ctr (US) | 0.06 | 144 |
| 6 | Germany | 0.11 | 505 | Beth Israel Deaconess Med Ctr (US) | 0.01 | 84 |
| 7 | UK | 0.08 | 404 | Harvard Univ (US) | 0.03 | 78 |
| 8 | Canada | 0.01 | 283 | Fudan Univ (CN) | 0.02 | 73 |
| 9 | Spain | 0.2 | 277 | Massachusetts Gen Hosp (US) | 0.03 | 70 |
| 10 | The Netherlands | 0.09 | 234 | Pfizer Inc (US) | 0.06 | 65 |
| Rank . | Country . | Centrality . | Count . | Institutes . | Centrality . | Count . |
|---|---|---|---|---|---|---|
| 1 | USA | 0.11 | 1,632 | Mem Sloan Kettering Cancer Centre (US) | 0.13 | 208 |
| 2 | France | 0 | 612 | Inst Gustave Roussy (FR) | 0.03 | 164 |
| 3 | Italy | 0.09 | 581 | Dana Farber Canc Inst (US) | 0.03 | 151 |
| 4 | China | 0 | 573 | Cleveland Clin (US) | 0.02 | 147 |
| 5 | Japan | 0 | 529 | Univ Texas MD Anderson Canc Ctr (US) | 0.06 | 144 |
| 6 | Germany | 0.11 | 505 | Beth Israel Deaconess Med Ctr (US) | 0.01 | 84 |
| 7 | UK | 0.08 | 404 | Harvard Univ (US) | 0.03 | 78 |
| 8 | Canada | 0.01 | 283 | Fudan Univ (CN) | 0.02 | 73 |
| 9 | Spain | 0.2 | 277 | Massachusetts Gen Hosp (US) | 0.03 | 70 |
| 10 | The Netherlands | 0.09 | 234 | Pfizer Inc (US) | 0.06 | 65 |
US, USA; FR, France; CN, China.
The output of articles and growth prediction of TKI for RCC at top five countries.
The output of articles and growth prediction of TKI for RCC at top five countries.
The 4,951 publications were issued by 647 core institutions, there were 913 links in the institution’s networks (destiny = 0.0345). Top 5 productive institutions account for 23.91% of the TKI for RCC worldwide (1,184 articles). Among them, Mem Sloan Kettering Cancer Centre (208 articles) contributed the most volumes, followed by Inst Gustave Roussy (164 articles), Dana Farber Canc Inst (151 articles), Cleveland Clin (147 articles), and Univ Texas MD Anderson Canc Ctr (144 articles), of which 4 institutions were located in the USA, and all of the top 5 institutions were run in developed members. In detail, Mem Sloan Kettering Cancer Centre with a higher research centrality (centrality >0.1) and larger outputs were becoming a pioneer in the field. More messages can be obtained in Table 1.
Analysis of Cited Journals
A total of 883 scholarly journals had made contributions to relevant research. This analysis aimed to identify the leading journals in this research field. Among the top 10 cited journals (Table 2), the most one was the Journal of Clinical Oncology (3,984 times), followed by the New England Journal of Medicine (3,771 times), Clinical Cancer Research (2,789 times), and Annals of Oncology (2,450 times). Among them, the New England Journal of Medicine, Clinical Cancer Research, and British Journal of Cancer had the higher centrality (centrality >0.1), depicting these three journals had a higher degree of research focus in TKI for the RCC field. In terms of the impact factor (IF), the Lancet (202.731), New England Journal of Medicine (176.079), and Lancet Oncology (54.433) have a higher impact than the others. The top ten co-cited journals were all classified in Q1 (the top 25% of the IF distribution).
The top 10 co-cited journals about TKI for RCC
| Rank . | Cited journal . | Citations . | If . | Centrality . | Q . | Country . |
|---|---|---|---|---|---|---|
| 1 | Journal of Clinical Oncology | 3,984 | 50.717 | 0.2 | Q1 | USA |
| 2 | The New England Journal of Medicine | 3,771 | 176.079 | 0.14 | Q1 | USA |
| 3 | Clinical Cancer Research | 2,789 | 13.801 | 0.19 | Q1 | USA |
| 4 | Annals of Oncology | 2,450 | 51.769 | 0.09 | Q1 | UK |
| 5 | The Lancet | 2,224 | 202.731 | 0.03 | Q1 | UK |
| 6 | The Lancet Oncology | 2,128 | 54.433 | 0.03 | Q1 | USA |
| 7 | Cancer Research | 1,911 | 13.312 | 0.22 | Q1 | USA |
| 8 | Cancer,Cancer-Am Cancer Soc | 1,868 | 6.921 | 0 | Q1 | USA |
| 9 | British Journal of Cancer | 1,867 | 9.075 | 0.12 | Q1 | UK |
| 10 | European Urology | 1,776 | 24.267 | 0.01 | Q1 | The Netherlands |
| Rank . | Cited journal . | Citations . | If . | Centrality . | Q . | Country . |
|---|---|---|---|---|---|---|
| 1 | Journal of Clinical Oncology | 3,984 | 50.717 | 0.2 | Q1 | USA |
| 2 | The New England Journal of Medicine | 3,771 | 176.079 | 0.14 | Q1 | USA |
| 3 | Clinical Cancer Research | 2,789 | 13.801 | 0.19 | Q1 | USA |
| 4 | Annals of Oncology | 2,450 | 51.769 | 0.09 | Q1 | UK |
| 5 | The Lancet | 2,224 | 202.731 | 0.03 | Q1 | UK |
| 6 | The Lancet Oncology | 2,128 | 54.433 | 0.03 | Q1 | USA |
| 7 | Cancer Research | 1,911 | 13.312 | 0.22 | Q1 | USA |
| 8 | Cancer,Cancer-Am Cancer Soc | 1,868 | 6.921 | 0 | Q1 | USA |
| 9 | British Journal of Cancer | 1,867 | 9.075 | 0.12 | Q1 | UK |
| 10 | European Urology | 1,776 | 24.267 | 0.01 | Q1 | The Netherlands |
IF, impact factor; Q, quartile in category.
Analysis of Authors and Cited-Authors
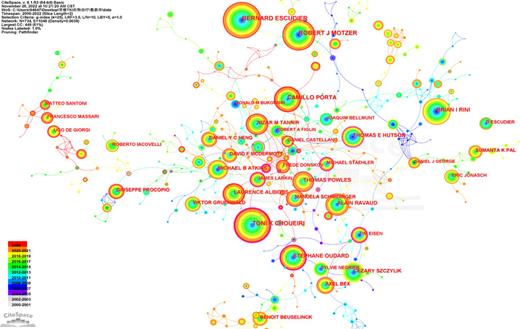
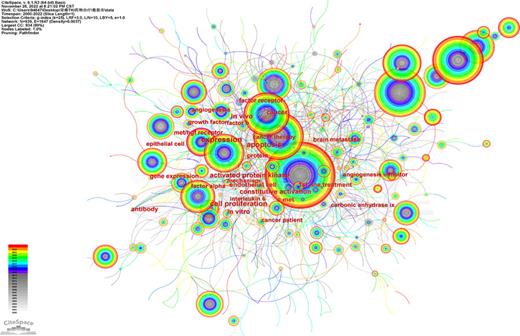
Author co-occurrence analysis is used to identify the most influential authors and assess the cooperation ability between different teams. A total of 734 core authors made most of the contribution to the research outputs. The author’s collaboration network map is displayed in Figure 6 with 734 nodes and 1,048 lines (destiny = 0.0039). Close internal links were observed between different authors on the map. The 10 most productive authors are displayed in Table 3. Escudier B, from Institut Gustave Roussy, published the most papers (141 publications), followed by Choueiri TK (129 publications), Motzer RJ (123 publications), Porta C (93 publications), and Rini BI (90 publications). Of the top 10 authors, 4 were from the USA, 4 were from France, and all authors were from developed countries. Developed countries have made the leading contribution in this area. The co-cited authors’ network visualization map is shown in Figure 7. The size of the circles represents the number of articles published, the larger the nodes the more the co-cited frequency. The links describe the degree of communication and interactions between authors. The most co-cited author was Motzer RJ (3,274 citations), followed by Escudier B (2,211 citations), Rini BI (2,062 citations), Choueiri TK (1,252 citations), and Sternberg CN (813 citations) (Table 3).
The top 10 authors and cited authors contributed to TKI for RCC
| Rank . | Author . | Centrality . | Count . | Cited author . | Centrality . | Count . |
|---|---|---|---|---|---|---|
| 1 | Escudier B | 0 | 141 | Motzer RJ | 0.73 | 3,274 |
| 2 | Choueiri TK | 0.12 | 129 | Escudier B | 0.72 | 2,211 |
| 3 | Motzer RJ | 0.06 | 123 | Rini BI | 0.23 | 2,062 |
| 4 | Porta C | 0.09 | 93 | Choueiri TK | 0.05 | 1,252 |
| 5 | Rini BI | 0 | 90 | Sternberg CN | 0 | 813 |
| 6 | Oudard S | 0.12 | 79 | Hudes G | 0.5 | 798 |
| 7 | Ravaud A | 0.01 | 71 | Heng DYC | 0.01 | 721 |
| 8 | Albiges L | 0 | 70 | Hutson TE | 0 | 598 |
| 9 | Powles T | 0.02 | 70 | Wilhelm SM | 0.43 | 461 |
| 10 | Hutson TE | 0.1 | 61 | Ljungberg B | 0.01 | 460 |
| Rank . | Author . | Centrality . | Count . | Cited author . | Centrality . | Count . |
|---|---|---|---|---|---|---|
| 1 | Escudier B | 0 | 141 | Motzer RJ | 0.73 | 3,274 |
| 2 | Choueiri TK | 0.12 | 129 | Escudier B | 0.72 | 2,211 |
| 3 | Motzer RJ | 0.06 | 123 | Rini BI | 0.23 | 2,062 |
| 4 | Porta C | 0.09 | 93 | Choueiri TK | 0.05 | 1,252 |
| 5 | Rini BI | 0 | 90 | Sternberg CN | 0 | 813 |
| 6 | Oudard S | 0.12 | 79 | Hudes G | 0.5 | 798 |
| 7 | Ravaud A | 0.01 | 71 | Heng DYC | 0.01 | 721 |
| 8 | Albiges L | 0 | 70 | Hutson TE | 0 | 598 |
| 9 | Powles T | 0.02 | 70 | Wilhelm SM | 0.43 | 461 |
| 10 | Hutson TE | 0.1 | 61 | Ljungberg B | 0.01 | 460 |
The cited author collaboration network of TKI for RCC from 2000 to 2022.
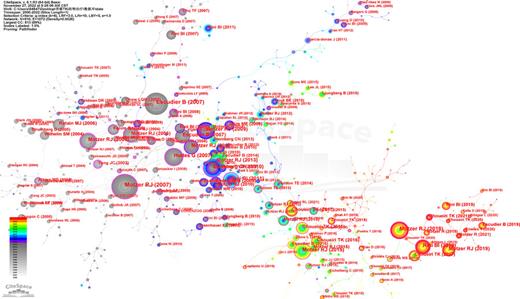
Analysis of Co-Cited References
Co-cited reference means that one or more references have been co-cited in one or more publications, of which to further explore the development trends. The corporation network map which had 910 nods and 1,072 links is depicted in Figure 8 (destiny = 0.0026). Most of the articles were cited 0∼10 times. Ten of the most frequently cited references [22‒31] (Table 4), six were reported by Motzer RJ [22, 24, 25, 28, 30, 31], the most highly cited paper was a multi-center, randomized, phase 3 trial about sunitinib that published in the New England Journal of Medicine in 2007 [22], with 696 citations. Of the remaining four articles, one was published by Escudier B [23] in 2018 with 608 citations, and the other by Rini BI [26] in 2019 with 373 citations. The last two were published by Sternberg CN [27] and Hudes G [29] in 2010 and 2007, respectively. All of the top ten cited references were in Q1. The topics covered in the highly cited papers are primarily about the progression of clinical intervention therapies and their safety.
The cited reference collaboration network of TKI for RCC from 2000 to 2022.
The top 10 highly cited articles and top 10 keywords of TKI for RCC
| Rank . | Articles . | Centrality . | Citations . | Keyword . | Centrality . | Count . |
|---|---|---|---|---|---|---|
| 1 | Motzer et al. [22] 2007 | 0.77 | 696 | renal cell carcinoma | 0.03 | 2,190 |
| 2 | Escudier et al. [23] 2007 | 0 | 608 | targeted therapy | 0 | 1,440 |
| 3 | Motzer et al. [24] 2018 | 0.13 | 459 | sunitinib | 0.03 | 1,222 |
| 4 | Motzer et al. [25] 2009 | 0.14 | 374 | interferon alpha | 0.05 | 1,185 |
| 5 | Rini et al. [26] 2019 | 0.12 | 373 | tyrosine kinase inhibitor | 0.02 | 1,105 |
| 6 | Sternberg et al. [27] 2010 | 0.79 | 370 | cancer | 0.16 | 1,005 |
| 7 | Motzer et al. [28] 2015 | 0.26 | 354 | survival | 0 | 820 |
| 8 | Hudes et al. [29] 2007 | 0.71 | 348 | sorafenib | 0.02 | 676 |
| 9 | Motzer et al. [30] 2008 | 0 | 319 | endothelial growth factor | 0.01 | 623 |
| 10 | Motzer et al. [31] 2019 | 0 | 318 | efficacy | 0 | 546 |
| Rank . | Articles . | Centrality . | Citations . | Keyword . | Centrality . | Count . |
|---|---|---|---|---|---|---|
| 1 | Motzer et al. [22] 2007 | 0.77 | 696 | renal cell carcinoma | 0.03 | 2,190 |
| 2 | Escudier et al. [23] 2007 | 0 | 608 | targeted therapy | 0 | 1,440 |
| 3 | Motzer et al. [24] 2018 | 0.13 | 459 | sunitinib | 0.03 | 1,222 |
| 4 | Motzer et al. [25] 2009 | 0.14 | 374 | interferon alpha | 0.05 | 1,185 |
| 5 | Rini et al. [26] 2019 | 0.12 | 373 | tyrosine kinase inhibitor | 0.02 | 1,105 |
| 6 | Sternberg et al. [27] 2010 | 0.79 | 370 | cancer | 0.16 | 1,005 |
| 7 | Motzer et al. [28] 2015 | 0.26 | 354 | survival | 0 | 820 |
| 8 | Hudes et al. [29] 2007 | 0.71 | 348 | sorafenib | 0.02 | 676 |
| 9 | Motzer et al. [30] 2008 | 0 | 319 | endothelial growth factor | 0.01 | 623 |
| 10 | Motzer et al. [31] 2019 | 0 | 318 | efficacy | 0 | 546 |
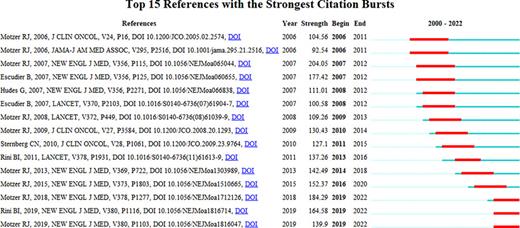
Furthermore, we performed references burst citation analysis. Figure 9 showed the top 15 relevant references. Motzer RJ reported on two studies that received significant attention from other researchers in 2006 and continued that attention for 5 years, ending in 2011. One of the articles was a multi-center phase 2 trial to test the activity of SU11248 (sunitinib malate) for metastatic ccRCC [32], about 40% of patients in this trial who were treated with SU11248 achieved promising responses. The outcomes supported the hypothesis that VEGF and PDGF receptor-mediated signaling were effective therapeutic targets in RCC. The other was another similar open-label, single-arm, multi-center clinical trial to confirm the antitumor efficacy of sunitinib for metastatic ccRCC, which was executed between 2004 and 2005 [33]. Of the 15 articles, 9 were published by Motzer RJ, it is obvious that Motzer RJ is one of the core authorities and has made a great contribution to promoting the research progress in this field. The remaining articles published by Escudier B, Sternberg CN, Rini BI, and others were also paid a lot of attention in the corresponding period.
The top 15 references with the strongest citation bursts in the co-citation network.
The top 15 references with the strongest citation bursts in the co-citation network.
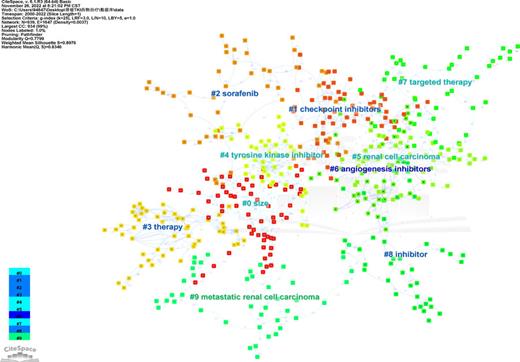
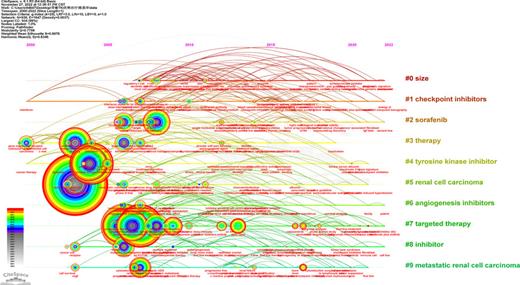
Analysis of Co-occurring Keywords and Cluster High-central keywords, and high-frequency keywords are considered hot topics in the research. Through keyword co-occurrence analysis and burst detection, we can better identify the changing trend of research topics over time. Co-occurrence network map, as shown in Figure 10, had 939 nods with 1,647 links (destiny = 0.0037). The most popular keywords were “renal cell carcinoma,” “targeted therapy,” “sunitinib,” “interferon alpha,” and “tyrosine kinase inhibitor” (Table 4). In addition, we visualized clusters of keywords by using a network map (Fig. 11). Cluster #0 “size” was the largest cluster, followed by “checkpoint inhibitors” (cluster #1), and “sorafenib” (cluster #2). Based on the timeline view of the keyword clusters (Fig. 12), several research directions, including “checkpoint inhibitors” (cluster #1), “sorafenib” (cluster#2), “angiogenesis inhibitors” (cluster #6), and “targeted therapy” (cluster #7) were currently the high-frequency research focus in this field.
The timeline view of the keywords in articles related to TKI for RCC treatment.
Analysis of Burst Keywords
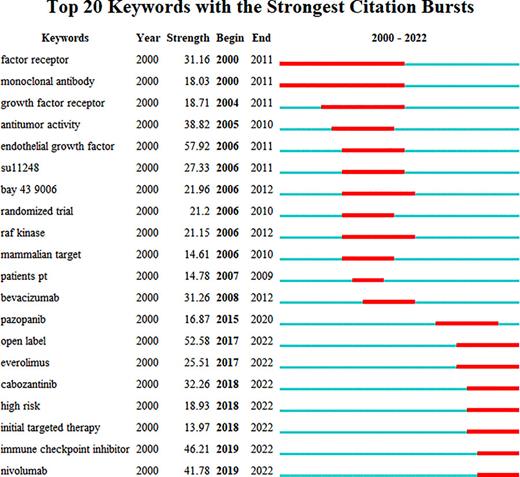
We used CiteSpace software to detect burst keywords in different periods. Figure 13 reflected the top 20 keywords with the strongest citation bursts. In particular, the keywords that appeared to burst in the first decade were “factor receptor” (burst strength is 31.6), “monoclonal antibody” (burst strength is 18.03), “antitumor activity” (burst strength is 38.82), “endothelial activity” (burst strength is 57.92), “su11248” (burst strength is 27.33), “raf kinase” (burst strength is 21.15), and “bevacizumab” (burst strength is 31.26). “Pazopanib” (burst strength is 16.87), “everolimus” (burst strength is 25.51), “cabozantinib” (burst strength is 32.26), “initial targeted therapy” (burst strength is 13.97), “immune checkpoint inhibitor” (burst strength is 46.21), and “nivolumab” (burst strength is 41.78) burst at the second decade, and almost all of them were still in the research focus now.
The analysis of burst keywords in articles related to TKI for RCC treatment.
Discussion
CiteSpace was used to analyze the knowledge base and research hot spots of TKI for RCC treatment in the WoSCC database from 2000 to 2022, several valuable facts were revealed in the report. First of all, there was a rapid increase in enthusiasm for research in this area. During the study period, papers issued in relevant journals expressed a steady increase worldwide. In 2021, 403 articles were published, and there is no hesitation in predicting that the 2022 production will be entirely parallel to or no less than 2021.
Additionally, the bibliometric analysis provided a multidimensional view of global trends over time, including countries, institutions, authors, and journals. As a result of a greater focus on and financial support for related research than in other regions, the USA was the leading contributor in this specific area. It was mainly driven by US scientists, as evidenced by the number of publications and the number of citations. The other four major contributors were France, Italy, Peoples R China, and Japan. France and Italy, as developed and European members, have a strong collaboration with each other. We could also get the signal from Figure 3 that there was a close collaboration among European members, most of them had a positive financial condition, including UK, Germany, Poland, Italy, France; it is worth promoting the cooperation mode in these counties to other areas like Asia and Africa. There was a natural correlation between the country’s outputs and the institutions. As mentioned above, in terms of publication production and citations, the USA was the leading country. Accordingly, eight of the top ten highest-producing institutions were from the USA, while the others were from France and China (Table 2). Mem Sloan Kettering Cancer Centre, Inst Gustave Roussy, and Dana Farber Canc Inst were the most influential institutions based on their total output numbers. Besides, among these ten institutions, six were cancer research centers, two were universities, and the other two were polyclinics.
For co-authorship analysis, Escudier B from Institut Gustave Roussy, Choueiri TK from Dana Farber Canc Inst, and Motzer RJ from Mem Sloan Kettering Cancer Centre reported the most articles, taking leadership roles in the field. Moreover, they actively collaborated to maximize regional advantages and increase academic impact. Most of Escudier B’s work was devoted to uncovering the secrets of TKI-targeted agents, like sunitinib, pazopanib, and cabozantinib. In 2009, he reported on a study of sunitinib for patients with cytokine refractory metastatic RCC, sunitinib 37.5 mg once-daily dosing regimen indicated a manageable safety profile in this randomized clinical trial. Objective response rates (ORRs) were 20% in a 7.2-month median response duration. Median PFS and OS were 8.2 and 19.8 months, respectively [34]. In 2014, the PISCES study, a double-blind and cross-over trial that assessed treatment preference for pazopanib versus sunitinib was reported [35]. Patients with metastatic RCC were randomly assigned to pazopanib 800 mg per day for 10 weeks, a 2-week washout, and then sunitinib 50 mg per day for 10 weeks, or the reverse sequence. No disease progression was observed in either group before the crossover, and patient preference in the pazopanib track was over than sunitinib because of its higher quality of life and safety. Escudier B reported another study about cabozantinib versus everolimus for metastatic RCC in 2018 [36]. The outcomes in cabozantinib treatment were associated with higher PFS, OS, and ORR, these results showed a potential alternative treatment for patients who had bone metastases. Choueiri TK, another leading investigator, has reported on several clinical studies of different drugs in metastatic RCC. Some of his work includes cabozantinib versus everolimus, cabozantinib versus sunitinib, nivolumab plus ipilimumab, or other combinations. In these studies, patients were divided into two or more different tracks, given different medications, then followed up and assessed for ORR, OS, and PFS [37‒39]. These studies assessed the clinical efficacy and safety profile of TKI drugs, advancing the development of related studies. Motzer RJ from the USA, whose researches include nivolumab plus cabozantinib versus sunitinib, nivolumab versus everolimus for metastatic RCC, etc [28, 30, 40]. Correspondingly, these authors were at the top of the cited authors list.
All of the top 10 cited journals were high IF members (defined as greater than 5,000) and were all classified in Q1. Interestingly, among the 10 journals, 7 were oncology JCR categories. The remaining, including the New EnglandJournal of Medicine, the Lancet, and European Urology, where the first two belong to medicine, general and internal and the last one is urology and nephrology, it appeared that oncology journals were more dedicated to advancing RCC than urology journals were. An area’s most cited articles could be a marker of its evolution. With 696 citations, the New England Journal of Medicine paper published in 2007 related to sunitinib and interferon alfa in metastatic RCC was the most influential [22]. In two earlier uncontrolled trials, sunitinib has already shown clinical activity in patients who had undergone previous cytokine therapy [32, 33]. As a result, the authors took a step further and reported the results of this randomized, phase 3 trial of sunitinib versus interferon alfa, while the interferon alfa was regarded as a first-line treatment for metastatic RCC in the first decade of the 21st century, and further concluded that the sunitinib track had longer PFS and higher ORR. There has been a lot of research conducted on TKI in the 2010s, in particular, including pazopanib, sunitinib, sorafenib, etc. The efficacy and safety profile of TKI drugs has been extensively tested. As a new milestone in the treatment history of RCC, immune CPIs had attracted increasing attention in recent years. PD-1 type therapy such as nivolumab has been approved for the treatment of RCC based on an OS benefit. Motzer RJ and his colleagues confirmed a higher OS and ORR in CPI plus track in a randomized phase 3 trial that compared nivolumab plus ipilimumab with sunitinib for previously untreated metastatic RCC [24], the 18-month ORR was 75% in CPI plus track and 60% in sunitinib track, respectively. The aforementioned study was published in 2018 and has received 459 citations so far, which was another milestone for RCC treatment.
In bibliometrics, keywords are high-level generalizations and concentration of the theme of the article, which can directly reflect the research hot spots in a specific area. Keywords co-occurrence analysis provides a typical overview of the core keywords, including renal cell carcinoma, sunitinib, interferon alpha, tyrosine kinase inhibitor, cancer, etc. A total of ten clusters were formed based on the keywords identified from the WoSCC, “checkpoint inhibitors” (cluster #1), “sorafenib” (cluster#2), “angiogenesis inhibitors” (cluster #6), and “targeted therapy” (cluster #7) were currently the high-frequency research focus, which represented RCC’s major research directions and frontiers. CPI and TKI were specifically mentioned, and based on the results obtained from the available data, the combination of these two agents was poised to move into the front-line setting. The KEYNOTE-146 trial demonstrated an encouraging antitumor activity and an improved ORR benefit for the combination of lenvatinib plus pembrolizumab (ORR 72.7% in naive track and 55.8% in the CPI-pretreated track) [41]. Moreover, CheckMate 214 confirmed the long-term benefit of ipilimumab plus nivolumab in metastatic RCC and poor-risk patients [42]. In CheckMate 016 study, although higher toxicities were observed in standard doses of sunitinib or pazopanib plus nivolumab, the evidence showed the relevant response was not adversely affected, and OS outcomes were noteworthy [43]. This finding confirmed that the success of the combination can be accessed, and that all that was required was simply a careful selection of the anti-angiogenic components and their dosage. More closely, in the recent guideline of the European Association of Urology, TKI plus CPI was recommended as the first line in advanced RCC, and dual immunotherapy of CPI plus CPI such as ipilimumab plus nivolumab was also highly recommended [44, 45]. Additionally, keyword-term burst detection was considered an indicator of research frontiers or emerging trends over time. As can be seen from Figure 13, in the first decade of the 21st century, researchers focused more on the exploration and research of kidney cancer-related signals like angiogenesis factors and found TKI agents such as sunitinib and axitinib, relevant clinical trials had a surge in the 2010s. However, in the following decade, especially after 2015, immune CPIs agents like PD-1 and PD-L1 gradually made their way into the research center. In particular, keyword citation burst of CPI agents appeared in 2019 and continued. TKI plus CPI is now the mainstream therapy for RCC.
There are some limitations to our study that need to be considered. On one hand, the WoSCC database was the only source for articles identified, and other sources such as PubMed and Embase were inadvertently omitted. On the other hand, due to our selective analysis of the information, some key points and details may have been overlooked. The data analysis in this study was conducted by software, the machine algorithm might also produce unnoticed biases.
Conclusion
We used CiteSpace to analyze the knowledge base and research hot spots of TKI for RCC treatment during the past 2 decades. Presently, publications related to TKI for RCC treatment are growing exponentially and citations are increasing annually, research passion in this area is growing rapidly. Meanwhile, we identified the leading countries, institutions, and prestigious researchers in the field, and analyzed seminal journals and representative literature. Immune CPIs and targeted therapies, along with therapeutic progression of RCC, are potential research hot spots. Immune CPIs plus angiogenesis inhibitors, with plenty of relevant clinical trials undergoing, are now paid a lot of attention to. Notably, European members had close cooperation, while different geographical locations like Africa and Asia did not; cooperation between different institutions and different authors was stable in the same country. International cooperation should be strengthened to increase the results of research. Wider and deeper cooperation is expected in the future.
Acknowledgments
We would like to thank the Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
Conflict of Interest Statement
The authors have no relevant financial or non-financial interests to disclose.
Funding Sources
This article was supported by the National Natural Science Foundation of China (No.82272840) and the National Natural Science Foundation of Guangdong, China (2021A1515010129).
Author Contributions
Jinbin Xu and ZhanSen Huang shared the first authorship and drafted the manuscript. JinMing Di designed the study, contributed to the interpretation of results and critical revision of the manuscript for important intellectual content, approved the final version of the manuscript, and was the study guarantor. ShunTian Gao and GengGuo Deng collected data. Jinbin Xu analyzed the data. All authors have read and approved the final manuscript.
References
Additional information
Jinbin Xu and Zhansen Huang contributed equally to this work and should be considered as co-first authors.