Dear Editor,
Pure seminoma accounts for about 46–60% of all testicular germ cell tumors [1]. The American Cancer Society estimated that the incidence of testicular cancer for 2022 is about 9,910 cases and the 5-year relative survival of testicular cancers was approximately 95% (2010–2016) [2]. More than 85% of patients present with stage I seminoma; management of stage I seminoma is generally by radical orchiectomy followed by either surveillance alone or further adjuvant radiotherapy or chemotherapy [3]. In an effort to minimize the toxicities and financial burden of overtreatment while preserving oncologic control, surveillance is preferred over adjuvant treatment by the National Comprehensive Cancer Network (NCCN) for patients with stage I seminoma [4]. In this study, we aimed to explore the oncologic benefits of adjuvant therapy following radical orchiectomy compared to surveillance alone in patients with stage I seminoma.
We retrospectively analyzed the SEER database between 2000 and 2018 [5]. We included patients with testicular cancer, selecting those with anaplastic or NOS seminoma. We used the “site recode WHO ICD-O-3” variable to select testis and the “histology recodes – broad groupings” variable to select patients with stage I seminoma and divided them into three cohorts: orchiectomy alone (surveillance), orchiectomy and chemotherapy, or orchiectomy and radiotherapy. We performed a Kaplan-Meier analysis to present the survival probabilities of patients according to the treatment regimen; statistical significance was assessed by the log-rank test. The Cox regression model was performed to calculate hazard ratios. p value <0.05 was considered statistically significant. We used R version 4.1.2 to perform the analyses.
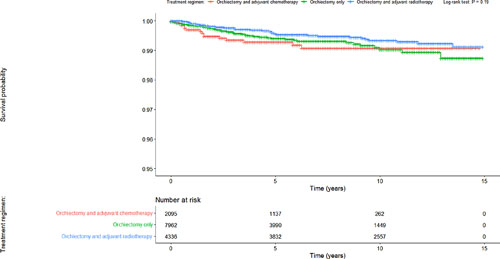
The analysis included 14,393 stage 1 seminoma patients of whom 7,962 (55.3%) were treated with orchiectomy only, 2,095 (14.6%) with orchiectomy and adjuvant chemotherapy, and 4,336 (30.1%) with orchiectomy and adjuvant radiotherapy. Cancer-specific survival (CSS) did not differ significantly among the treatment regimens (p = 0.19) (Fig. 1). Median follow-up was 82 months (range: 0–179). The Cox proportional hazards model showed insignificant hazard ratios for patients treated with adjuvant chemotherapy or radiotherapy compared to patients managed with surveillance alone (Table 1).
Kaplan-Meier survival curves for patients with stage I seminoma according to the treatment regimen.
Kaplan-Meier survival curves for patients with stage I seminoma according to the treatment regimen.
Univariate cox proportional hazards model
| Treatment regimen . | Hazard ratio . | 95% CI . | p value . |
|---|---|---|---|
| Orchiectomy only | Reference group | ||
| Orchiectomy and adjuvant chemotherapy | 1.22 | (0.68–2.19) | 0.50 |
| Orchiectomy and adjuvant radiotherapy | 0.71 | (0.44–1.15) | 0.16 |
| Treatment regimen . | Hazard ratio . | 95% CI . | p value . |
|---|---|---|---|
| Orchiectomy only | Reference group | ||
| Orchiectomy and adjuvant chemotherapy | 1.22 | (0.68–2.19) | 0.50 |
| Orchiectomy and adjuvant radiotherapy | 0.71 | (0.44–1.15) | 0.16 |
Our findings demonstrate that CSS was favorable overall for patients with stage I seminoma regardless of management strategy (surveillance or adjuvant treatment). These findings are concordant with those of Kollmannsberger et al. [6] in which adjuvant chemotherapy or radiotherapy did not increase CSS in stage 1 seminoma patients. In another study of the National Cancer Database, among 8,686 patients with stage I seminoma followed between 2004 and 2016, the utilization of surveillance increased substantially from 39.8% in 2004 to 86.8% in 2016, while the use of radiotherapy declined from 59.7% to 4.6% [7]. The survival outcomes in our study are comparable between the surveillance group and the radiotherapy group based on the lack of statistical significance seen. Also, overall, the survival for this population is very favorable, as evidenced by the long-term survival seen in the surveillance group. However, treatment with radiotherapy approximately doubled the risk of secondary solid malignancies in survivors of seminoma [8]. While long-term radiation exposure and IV contrast administration raise inherent long-term survivorship concerns for a relatively young population of patients who would be conceivably surveilled for decades, the recent TRISST trial showed that MRI could be used as an alternative to CT for testicular cancer surveillance based on its noninferiority [9]. Thus, although limited by the lack of information surrounding radiotherapy dosage and chemotherapy regimens used, our findings support a strategy of surveillance in patients with stage I seminoma in order to avoid the toxicities and financial burden associated with adjuvant chemotherapy or radiotherapy.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding to declare.
Author Contributions
Anas Elgenidy, Yousef Tarek Sonbol, and Ahmed O. Elmehrath: writing, analysis, acquisition of data, and analysis and interpretation of data. Ahmed M. Afifi and Nirmish Singla: critical revision, drafting, and study supervision.
References
Additional information
Anas Elgenidy, Yousef Tarek Sonbol, and Ahmed O. Elmehrath contributed equally to this work.