Abstract
Objectives: This study aimed to evaluate the cumulative incidence of overall and severe radiation cystitis following external beam radiation therapy for prostate cancer and investigate the clinical factors predictive of radiation cystitis. Methods: This retrospective study comprised 246 patients who received external beam radiation therapy for localized or locally advanced prostate cancer between 2013 and 2016 in our institution. Of these, 189 received primary radiation therapy and 57 received adjuvant/salvage radiation therapy. Radiation cystitis was recorded using the Common Terminology Criteria for Adverse Events version 5.0 definition, and severe radiation cystitis was defined as grade 3 or higher. All medical records were reviewed to calculate the cumulative incidence of radiation cystitis. Univariate and multivariate Cox regression analyses were used to evaluate its association with clinicopathologic features. Results: The median follow-up period after radiation therapy was 56 months (range 5–81). The 5-year cumulative incidence of radiation cystitis and severe radiation cystitis was 16.2% and 3.0%, respectively. Multivariate analyses identified radiation therapy in the adjuvant/salvage setting was the sole risk factor associated with the development of radiation cystitis (hazard ratio: 2.75, p = 0.02). Conclusions: Radiation therapy in the post-prostatectomy setting was associated with increased risk of radiation cystitis compared with radiotherapy as the primary treatment.
Introduction
Prostate cancer (PCa) is one of the most common cancers, especially in developed countries. For nonmetastatic localized PCa, radical prostatectomy and radiation therapy (RT) have been established as comparable treatment options [1‒3]. Therefore, most patients consider each treatment option in terms of possible complications.
RT has been frequently applied not only as the primary treatment for localized or locally advanced PCa but also as the adjuvant/salvage therapy following radical prostatectomy. Although the innovation of radiation modalities has contributed to improvement of tumor control and safety, patients must be informed of the potential risks of genitourinary and gastrointestinal toxicities [4].
Radiation cystitis (RC) is a hemorrhagic type of cystitis caused by RT to the pelvis. The higher the radiation exposure, the greater the risk of developing RC. In addition, most RC cases have delayed onset times which can occur as late as 20 years after RT [5, 6]. The true cumulative incidence of RC has been a matter of controversy, ranging from 9% to 25% in previous reports [7, 8]. The pathologic characteristic of RC is a progressive obliterative endarteritis, inducing a bladder hypoperfusion, which results in atrophy and fibrosis, and subsequently necrosis of the bladder mucosa and hematuria [6].
The management of RC has been challenging and not established. Persistent hemorrhage from the bladder mucosa, if irreversible and progressive, would make consideration of transurethral coagulation or hyperbaric oxygen therapy [9‒11]. Furthermore, refractory to these treatments would require urinary diversion [9]. Hemorrhagic anemia and infections with RC can even have fatal consequences.
Although several papers have reported the incidence of genitourinary and gastrointestinal toxicities as adverse events of RT [4, 12‒15], only a few studies have focused on RC and investigated its potential risk factors. In these studies, smoking and antithrombotic treatment have been shown as substantial risk factors for RC [8, 16, 17]. Intensity-modulated radiation therapy (IMRT), which has revolutionized radiation oncology in recent years, is expected to reduce radiation exposure to the periprostatic organs, especially the bladder and rectum [4]. It is of use identifying the incidence of RC in the era of IMRT. Therefore, in this retrospective study, we investigated the cumulative incidence of RC, as well as predictive risk factors for RC in patients who had undergone external beam radiation therapy (EBRT) for PCa.
Patients and Methods
Study Population
This study was approved by the institutional review board. Between 2013 and 2016, 269 patients underwent RT for nonmetastatic PCa at the University of Tokyo Hospital. Of these, we excluded the patients who underwent brachytherapy with or without EBRT or were followed less than 3 months, leaving 246 patients (91%) available for analysis.
Medical reports were reviewed, and the following clinical parameters were collected: age, gender, comorbidities including chronic hypertension and diabetes mellitus, administration of antithrombotic drugs, prostate-specific antigen (PSA) level at the diagnosis, clinical T stage, grade group, RT setting (primary or adjuvant/salvage), and radiation cumulative dose (Gy) of the RT.
RC was diagnosed by the occurrence of gross hematuria without other explainable causes such as infection, genitourinary malignancy, or drug-induced cystitis. Severity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, and severe RC was defined as grade 3 or higher.
Protocol of IMRT
According to the D’Amico risk classification [1, 2], EBRT as a primary RT was performed to the prostate only for the low-risk group, to the prostate and base of the seminal vesicle for the intermediate-risk group, and to the prostate and seminal vesicle for the high-risk group. The conventional schedule was 38 fractions of 2 Gy to a total of 76 Gy and reduced to 72 Gy in case of administration of antithrombotic drugs.
Indications of adjuvant RT were determined based on patients’ treatment preference and adverse pathological features including seminal vesicle invasion, extraprostatic extension with positive surgical margin, or accompanying Gleason pattern 5. Salvage RT was performed in case of PSA recurrence [1, 2]. The definition of the clinical target volume was determined according to Radiation Therapy Oncology Group (RTOG) consensus guidelines and previously described in detail [18]. Patients treated with adjuvant RT received 62 Gy in 31 fractions, and those with salvage RT received 66 Gy in 33 fractions, if any, with a boost to the recurrent lesion, where having been detected by magnetic resonance imaging.
Statistical Analysis
Kaplan-Meier analysis was used to estimate cumulative incidence of RC and severe RC. Cox proportional hazards regression models were used for univariate and multivariate analyses. Statistical computations were carried out using JMP 13.2.1 (SAS Institute, Cary, NC, USA). Significance was set at a p value of p < 0.05.
Results
Patient Characteristics
Demographic features and tumor characteristics are shown in Table 1. The median follow-up time was 56 months (interquartile range, IQR, 47–64 months). More than three-quarters of the patients (n = 189, 77%) were primarily treated with EBRT, and the others (n = 57, 23%) were submitted to an adjuvant or salvage RT. Sixty-eight (27%) patients received antithrombotic therapy. Surgical pathology identified 28 patients with extraprostatic extension and 11 with seminal vesicle invasion. Forty-three cases had positive surgical margins. Ten patients received adjuvant RT and 47 underwent salvage RT due to PSA recurrence during follow-up.
All patients who received EBRT as initial treatment were treated with IMRT. On the other hand, of the patients who underwent adjuvant/salvage RT, 40 (70%) were treated with IMRT and 17 (30%) with three-dimensional conformal RT (3D-CRT).
Cumulative Incidence of RC
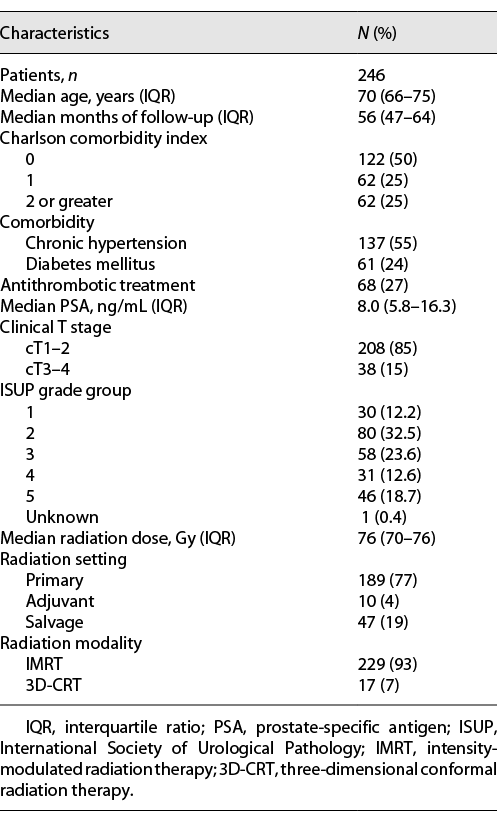
The 5-year cumulative incidence of RC and severe RC was 16.2% and 3.0%, respectively (Fig. 1). Of the 34 patients who developed RC, 19 had an unscheduled outpatient visit, and 6 of these patients required hospitalization for further treatment; blood transfusion was required in 2 (0.8%), and transurethral coagulation and hyperbaric oxygen therapy were performed in 1 (0.4%) and 3 (1.2%), respectively. The cumulative number of RC increased linearly after the latent phase of 1 year, and it was assumed that the risk of developing macrohematuria remained almost constant throughout the period after EBRT. Excluding 3D-CRT cases, the 5-year cumulative incidence of RC and severe RC was calculated to be 15.1% and 3.2%, respectively. On the other hand, the 5-year cumulative incidence of radiation proctitis was 11.4% in our cohort.
Cumulative incidence of radiation cystitis (a), severe radiation cystitis (b).
Risk Factors for RC
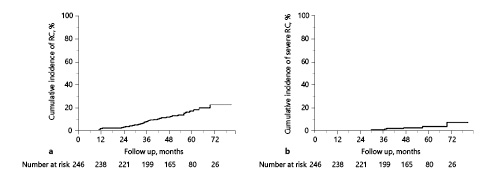
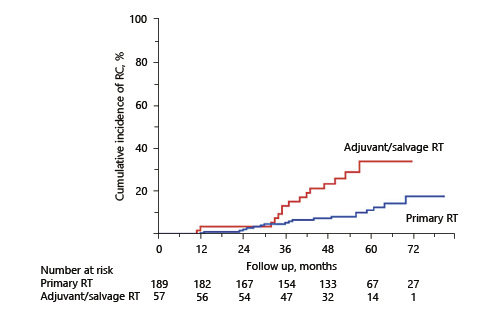
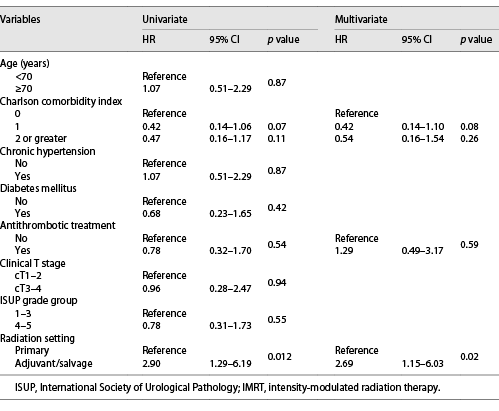
The associations of clinicopathologic factors with RC are shown in Table 2. Univariate analyses revealed Charlson comorbidity index and RT setting were significantly associated with the development of RC. Multivariate analyses identified RT setting as the sole significant factor associated with RC (hazard ratio: 2.75, p = 0.02). Antithrombotic therapy did not affect macrohematuria in this study. Similar results were obtained in subgroup analyses that excluded patients using modality other than IMRT (Table 3). Figure 2 shows the cumulative incidence of RC in the patients treated as primary RT and adjuvant/salvage RT, respectively. The 5-year cumulative incidence of RC was 11.1% in the primary RT group and 33.5% in the adjuvant/salvage RT group. On the other hand, regarding radiation proctitis, Cox regression analysis revealed that RT setting was not a significant predictive factor (p = 0.12). Furthermore, we could not identify any statistically significant factor in the development of severe RC.
Univariate and multivariate Cox proportional hazards regression analyses of the association of clinicopathologic variables with radiation cystitis
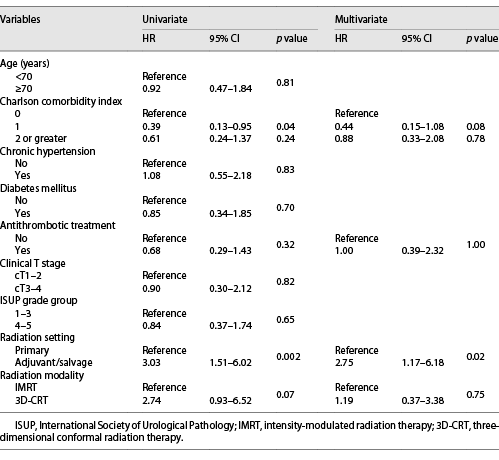
Univariate and multivariate Cox proportional hazards regression analyses of the association of clinicopathologic variables with radiation cystitis in patients treated with IMRT
Cumulative incidence of radiation cystitis in the patients treated as primary RT and adjuvant/salvage RT.
Cumulative incidence of radiation cystitis in the patients treated as primary RT and adjuvant/salvage RT.
Discussion/Conclusion
In our study, the 5-year cumulative incidence of RC was 16.2%, consistent with the reported incidence of RC observed in previous studies [7, 8]. In addition, the 5-year cumulative incidence of severe RC was only 3.0%, and no case required urinary diversion. Nevertheless, the true cumulative incidence of RC, especially in the long term, is controversial. The reasons for this are as follows: different definitions for each study, long observation periods required due to long time to onset, high lost to follow-up rates, and use of different radiation modalities.
This low incidence of severe RC could be explained by the introduction of new radiation therapy techniques such as IMRT. With the advent of dose-escalating radiation therapy, it was presumed that radiation doses to target organs have increased, while decreasing to adjacent organs, resulting in less toxicity. It was reported that IMRT was associated with a significant reduction in acute gastrointestinal and genitourinary toxicity [19].
To our knowledge, this is the first study comparing RT settings for the development of RC, especially in the era of IMRT. Our results demonstrated that adjuvant/salvage RT had significantly higher risk for RC than primary RT in PCa patients. On the other hand, there was no correlation between the cumulative incidence of RC and age, clinical T stage, or antithrombotic therapy.
The most likely reason for RC to be more common in the adjuvant/salvage RT group was explained by prostate removal, which led to the bladder to be exposed to more radiation. The fact that the incidence of radiation proctitis did not differ between RT settings further substantiated this hypothesis.
The 5-year cumulative incidence in the adjuvant/salvage RT group was 33.5%, which was relatively high compared to cases previously reported from other countries [13‒15, 20], but most were grade 2 or lower. A single institution in Japan reported a 20.4% prevalence of RC in the adjuvant/salvage RT group undergoing IMRT [21]. However, it should be noted that this report calculated prevalence, not cumulative incidence, and the 5-year cumulative incidence might be higher than the reported proportion.
Racial differences may be involved in the high incidence of RC in the adjuvant/salvage RT group. In addition, there was a possibility that the bladder was exposed to more amount of radiation as a result of the inability to fully extend the bladder during irradiation because of postoperative urinary incontinence and loss of functional bladder capacity, although precise reasons remain unclear.
Stereotactic radiotherapy, which is currently used as a standard treatment for PCa in our institute, is a new modality and should be taken into consideration in future studies [22]. Information on the accurate and updated cumulative incidence of RC and its potential risk factors is important to properly advise patients about the possible adverse effects of RT. This study demonstrated that IMRT could be safely applied to the elderly, even if they underwent antithrombotic treatment. As life expectancy is extended and the number of patients with PCa is increasing, RT would be a more important treatment option.
In addition, it is a pressing matter to reduce the positive margin rate of localized PCa for urologists. Robot-assisted radical prostatectomy has become the standard treatment, and the positive margin rate has been significantly reduced compared to retropubic radical prostatectomy [23]. However, it was reported that the positive margin rate was still around 10% [23]. We must keep in mind that reducing postoperative PSA recurrence to avoid salvage RT is the best way to prevent the adverse effects caused by excessive RT. Furthermore, we should carefully consider the indication of adjuvant RT.
Our study was limited by its retrospective design and small sample size. Additionally, the study was considerably lacking in follow-up cases, and at the 5-year follow-up point, as less as one-third of the patients could be followed, which might lead to the increase of the error of the cumulative incidence. Finally, as cystoscopy was not necessarily required to diagnose RC in our study, the incidence of RC might be overestimated.
In conclusion, the 5-year cumulative incidence of RC and severe RC was 16.2% and 3.0%, respectively. This study suggested that RT in the post-prostatectomy setting would increase the risk of RC, although most of the cases were grade 2 or lower and resolved by conservative treatment.
Statement of Ethics
This study has been approved by the Ethics Committee at The University of Tokyo Hospital (Approval No. 3124) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent to participate and for publication was obtained from the patients.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This manuscript did not receive any funding.
Author Contributions
Conception or design of this work: K.M., Y.S., and H.K. Data acquisition: K.M., R.T., and H.Y. Interpretation of data for this work: K.M., Y.S., Y.A., Y.Y., M.N., T.K., D.Y., and M.S. Drafting the manuscript: K.M. Critically revising the manuscript: Y.S. and H.K. All authors reviewed the manuscript and approved the final version of this work.
Data Availability Statement
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants, but are available from the corresponding author (Y.S.) upon reasonable request.