Abstract
Introduction: The aim of this study was to implement our technique for the initial dissection of the inferior hypogastric plexus and protection of the autonomic nerve supply to the corpora cavernosa in laparoscopic radical cystoprostatectomy with an orthotopic ileal neobladder and report the initial outcomes. Methods: Eleven normally potent patients with preoperative cT2N0 bladder cancer who underwent bilateral nerve-sparing laparoscopic cystoprostatectomy performed by the same surgeon were selected from May 2018 to September 2020. In this procedure, the anterior part of the inferior hypogastric plexus was dissected first between the prehypogastric nerve fascia and rectal proper fascia medial to the distal ureter. Then the Denonvilliers’ fascia and the nerves around the prostate were preserved according to current intrafascial principles. The preliminary operative, oncologic, and functional results are presented. Results: The median follow-up duration was 18 months. We observed early and late complications in 5 patients, but none exceeded grade III. Of the 11 patients, ten gained daytime continence (90.9%), and 8 (72.7%) showed nocturnal continence at the last follow-up. Regarding postoperative potency, 10 of the 11 patients (90.9%) remained potent with or without oral medications, excluding one who had partial tumescence but did not follow our recommendations regarding medication use. No local recurrence or positive surgical margins were noted. Conclusion: In addition to emphasizing our cavernosal nerve-sparing procedure, this report on the precise dissection and protection of the inferior hypogastric plexus could be of clinical significance, providing potentially ideal short-term functional results.
Introduction
Radical cystectomy (RC) is the gold standard approach for patients with muscle-invasive bladder cancer or recurrent high-grade superficial tumors [1]. However, subsequent functional complications cannot be ignored, especially sexual dysfunction and urinary incontinence, which may cause long-term distress to patients and severely compromise their quality of life [2].
Soon after Walsh indicated that neurovascular bundle (NVB) damage was a significant cause of erectile dysfunction in men, a novel technique to preserve sexual function with cystectomy was developed [3]. Similar to prostatectomy, nerve-sparing cystoprostatectomy also implies NVB protection and led to some improvements in potency [4]. However, overall functional results have remained unsatisfactory. On the basis of a more precise recognition of the relevant basic neurofunctional anatomy, in order to overcome this limitation, some experts had developed prostate-, capsule-, or seminal vesicle (SV)-sparing techniques to minimize functional morbidity [4‒6]. Ideally, these approaches avoid manipulation of the inferior hypogastric plexus (IHP) and NVB in an innovative fashion relative to typical nerve-sparing techniques. However, it should be noted that organ-sparing approaches contradict the standard resection scope of RC and therefore may sacrifice oncologic results [7]. Therefore, the conventional theory is not enough to settle the issue of the nervi erigentes protection. It is necessary for us to explore a novel anatomical strategy to better locate and protect the nerves. In this article, we present the technique and preliminary results related to the initial dissection of IHP and protecting nerves under direct vision throughout the whole process.
Surgical Technique
The patient was placed in a 30° Trendelenburg tilt. A 5-port laparoscopic technique was used, with the camera port positioned above the umbilicus at the midline. In view of the camera, 10-mm and 12-mm ports were placed level with the umbilicus on both sides lateral to the rectus sheath, and two 5-mm assistant ports were placed on either side of the anterior superior iliac spine.
The innovation of our technique is to accurately dissect the plane of the IHP first before pelvic lymphadenectomy. Intraoperatively, we strived to preserve Denonvilliers' fascia (DVF) and performed intrafascial nerve sparing in the prostate. The key steps of the surgical technique are outlined below.
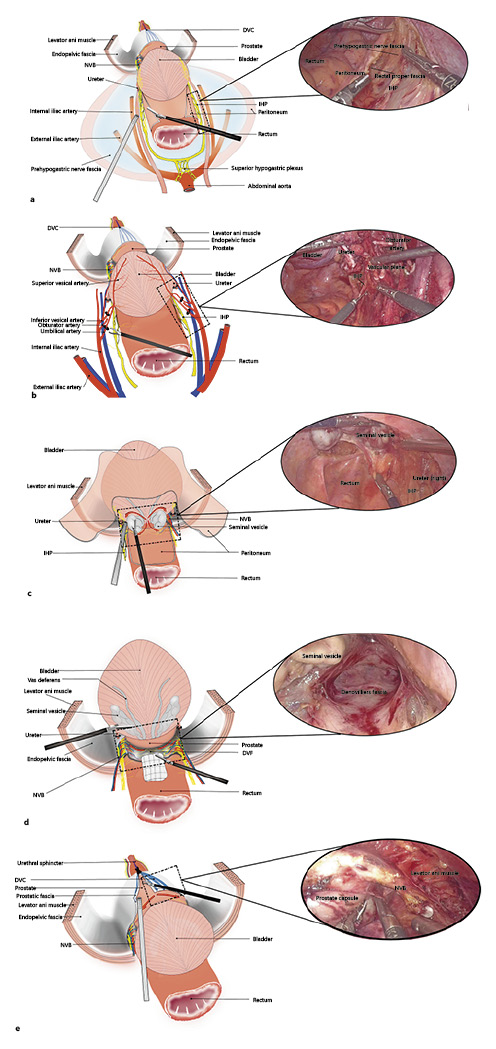
First, the right ureter was identified, and the covering peritoneum was cautiously opened. A plane between the peritoneum and the prehypogastric nerve fascia was created adjacent to the medial plane of the distal ureter. Dissection was performed caudally and inferiorly to the space between the prehypogastric nerve fascia and rectal proper fascia. Then, the anterior part of the IHP was precisely dissected lateral to the rectum. Thereafter, the prehypogastric nerve fascia covering the right ureter was opened and mobilized toward the ureterovesical junction where it was transected with a clip. The principal difference between both sides is that an avascular retroperitoneal plane of Toldt’s fascia is developed between the mesentery and parietal peritoneum on the left side of the sigmoid colon before left IHP dissection (Fig. 1a).
Subsequently, bilateral pelvic lymph node dissection (PLND) was performed. The boundaries included the bifurcation of the common iliac artery proximally, the bladder wall medially, the genitofemoral nerve laterally, and the inguinal ligament distally.
The bladder pedicles were the next focus of attention. The umbilical artery and superior bladder artery and vesical veins were progressively dissected and transected. As a result, the IHP was situated alone in the medial sagittal plane of these vessels. The vessels were clamped and transected away from the IHP plane under direct visualization (Fig. 1b).
Peritoneal reflection was incised to expose the SVs and vas deferens. The SVs were separated close to the prostate along the ventral aspect of the DVF. The SVs were dissected along their ventral and dorsal aspects from the medial side to the lateral side (Fig. 1c). The camera was flipped to face 30° upwards before commencing the posterior prostate dissection. A space was created between the prostate and DVF laterally to either the prostatic side or distally to the apex, attempting to leave the DVF in place (Fig. 1d).
The peritoneum lateral to the sagittal plane of the medial umbilical ligament was incised to access the Retzius space. A space between the bladder and anterior abdominal wall was created, extending the plane toward the endopelvic fascia bilaterally and caudally. Then, the umbilical ligament and urachus were resected, and the overlying fatty tissue around the prostate was cleaned up.
The visceral and parietal endopelvic fasciae up to the level of the fascial tendinous arch were meticulously separated. The dorsal venous complex was ligated with 2-0 sutures.
The prostatic fascia on the ventral side was incised before bilateral intrafascial dissection was performed, allowing for communication between this plane and the previously dissected posterior plane (Fig. 1e). Next, the prostatic vascular pedicles were secured with a Hemo-lock clip and sharply separated with cold scissors.
The prostatic capsule was kept close to dissect the prostatic apex, transverse the dorsal venous complex, and maximally preserve the urethral stump. Then, the urethra was transected at the apex, taking care to preserve the tissue adjacent to the membranous urethra.
Surgical steps. Inferior hypogastric plexus dissection (a); Gaining control of the bladder pedicles (b); Autonomic nerve protection in the SV area (c); Maintaining the integrity of the DVF (d); Incision of the ventral prostatic fascia to perform intrafascial dissection (e). VD, vas deferens.
Surgical steps. Inferior hypogastric plexus dissection (a); Gaining control of the bladder pedicles (b); Autonomic nerve protection in the SV area (c); Maintaining the integrity of the DVF (d); Incision of the ventral prostatic fascia to perform intrafascial dissection (e). VD, vas deferens.
Finally, the specimen was placed in an Endobag and extracted via an 8-cm infraumbilical midline incision. Then, a “W”-shaped neobladder was extracorporeally reconstructed with a 40-cm ileal segment and anastomosed intracorporeally with the urethra. Any hemorrhage or leakage was evaluated before leaving a pelvic drain in place.
Patients and Methods
Patient Selection
From March 2018 to September 2020, of the 66 cystectomies performed in male patients, 11 patients underwent nerve-sparing laparoscopic cystoprostatectomy with orthotopic urinary substitution. In addition to the usual indications for RC, these patients met several inclusion criteria for nerve-sparing cystectomy, such as an age younger than 65 years with a normal baseline 5-item International Index of Erectile Function (IIEF-5) score of 22–25, bladder cancer stage ≤ pT2-N0-M0 based on a preoperative assessment, and a strong desire to preserve potency and continence. Patients with tumors growing into the bladder neck or urethra were excluded.
All patients underwent a thorough preoperative assessment, including routine blood tests, blood biochemistry, urinalysis, and abdominopelvic computed tomography or magnetic resonance imaging. Patient demographics and preoperative, operative, and postoperative data were analyzed. Early (<30 days) and late (>30 days) comorbidities were also recorded and graded according to the Clavien-Dindo system [8].
Follow-Up
The patients were followed-up every 3 months for year 1 and every 6 months thereafter. Follow-up consisted of imaging examinations, cystoscopy, and a validated questionnaire regarding sexual function and continence. Patients who postoperatively achieved adequate nightly erections or erections for the completion of intercourse were considered to be potent. The timing of the first appearance of these potency indicators was defined as recovery. Continence was defined as being pad free or requiring 1 pad for safety.
Results
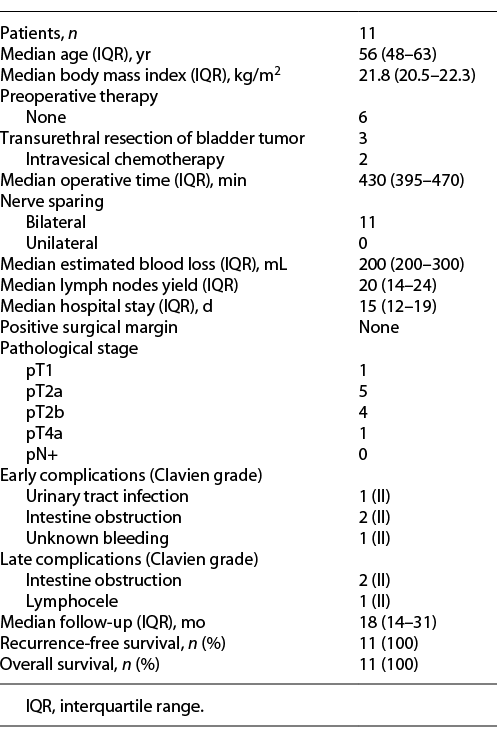
All of the procedures were completed successfully without any major intraoperative complications, and bilateral nerve-sparing cystoprostatectomy was performed on all patients. The median patient age, body mass index, operative time, and lymph node yield were 56 years (interquartile range [IQR]: 48–63), 21.8 kg/m2 (IQR: 20.5–22.3), 430 min (IQR: 395–470), and 20 (IQR: 14–24), respectively. The overall median estimated blood loss was 200 mL (IQR: 200–300), and the duration of postoperative hospital stay was 15 days (IQR: 12–19). Early and late complications occurred in 4 and 2 patients, respectively, but none were high-grade complications (Table 1).
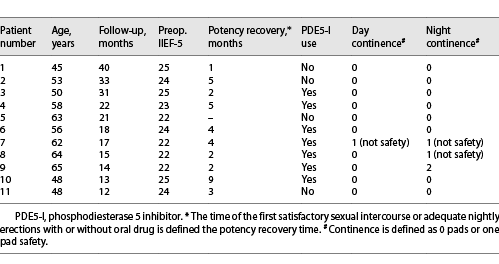
In terms of pathology, 1 patient had pT1 cancer, 9 had pT2a/T2b cancer, and 1 had pT4a cancer due to prostate infiltration that could not be detected by preoperative imaging or cystoscopy. The surgical margins and lymph nodes were all negative for metastasis. The median postoperative follow-up time was 18 months (IQR: 14–31), and the recurrence-free survival rate was 100%. At the last follow-up, daytime and nocturnal continence was preserved in 90.9% and 72.7% of patients, respectively. Of the 11 patients, 10 recovered potency within 9 months postoperatively: 3 showed spontaneous erections, and the remaining 7 achieved an erection with the aid of sildenafil. One patient over the 1-year follow-up demonstrated partial tumescence, but this patient did not follow our advice on the use of medication (Table 2).
Discussion
The desired goal of surgical intervention for bladder cancer is to achieve an oncologic cure while preserving continence and sexual function. For men, there are 4 sparing techniques used to improve functional results: nerve sparing, prostate sparing, capsule sparing, and SV sparing; no specific technique has demonstrated to be preferential [1]. However, to our knowledge, the best potency rate for dozens of patients who underwent nerve-sparing surgery in a single institute was 64% [9], while it was 91.6% for organ-sparing surgery [10]. Hence, the nerve-sparing approach deserves further exploration.
In contrast to nerve-sparing radical prostatectomy, we asked whether the nerves need to be further protected when a larger surgical area is required for RC. After thoroughly examining the development of potency-sparing RC, there are two methods for NVB protection. One is a nerve-sparing approach performed close to the nerves and the other is an organ-sparing technique implemented away from the NVB distribution areas. Although avoiding NVB manipulation should render perfect postoperative functional effects, this is not the case. Some reports have indicated that some patients did not respond with potency recovery [11, 12]. We speculate that although the organ-sparing approach perfectly retains the NVB, this approach may damage the IHP where the NVB originates. The IHP and erective nerves are at risk during standard medial dissection in the area of the internal iliac artery and toward the bladder wall; they are also at risk at their origin in the presacral area and medial to the common iliac vessels during PLND [13]. Therefore, nerve sparing should emphasize comprehensive protection starting from the most vulnerable part, that is, IHP which NVB originates.
In our process, We first accurately locate and dissect the IHP. This allows us to perform various measures under direct visualization of the IHP and NVB, such as SVs dissection and prostate ressection. We herein adopted the pelvic autonomic nerve preservation method in colorectal surgery to address IHP positioning and dissection. In terms of perirectal structure, two fasciae can be identified between the rectal proper fascia and sacral periosteum [14]. Adjacent to the rectal proper fascia lays the prehypogastric nerve fascia and the other is the presacral fascia, as demonstrated by Kigunasa through histologic examination [15]. Sharp dissection medial to the prehypogastric nerve fascia allows for precise planes between the IHP and rectal proper fascia, which not only meets the total mesorectal excision standard but also preserves the pelvic autonomic nerve (Fig. 2a).
a The relationships among the ureter, pelvic autonomic nerve and fasciae. b Autonomic nerve dissection of the pelvic organs (the arrow indicates the line of dissection when performing nerve-sparing cystoprostatectomy). PANP, pelvic autonomic nerve preservation; TME, total mesorectal excision.
a The relationships among the ureter, pelvic autonomic nerve and fasciae. b Autonomic nerve dissection of the pelvic organs (the arrow indicates the line of dissection when performing nerve-sparing cystoprostatectomy). PANP, pelvic autonomic nerve preservation; TME, total mesorectal excision.
Regarding RC, the relationship between autonomic nerves and fascia should be delineated in another way. To achieve this, novel comprehension of the renal fascia anatomy must first be gained. As Coffey pointed out, the renal fascia is contiguous with the pelvic fascia but has various names in different regions [16]. In fetal and adult cadaveric research [17, 18], the anterior and posterior renal fascia run downward to form a chamber-like structure that carries the hypogastric nerves and ureters to their terminations in the pelvis. In response to the abovementioned total mesorectal excision standard and pelvic autonomic nerve preservation cognition in rectal surgery, we specifically adopted the terminology “prehypogastric nerve fascia,” as described by Kigunasa et al. [15], to refer to the structure that extends from the renal fascia to the perirectum. This fascia covers the bilateral hypogastric nerve and IHP and then extends anterolaterally to connect with the lateral continuation of the DVF [14]. Based on this cutting-edge anatomical theory, we progressively separated the structures around the pelvic ureter, and the dissection plane medial to the ureter can lead to the space between the continuous renal fascia and rectal proper fascia (Fig. 2a).
An unobstructed view of the IHP after precise dissection would reduce the risk of damage to the pelvic autonomic nerve during PLND. In our initial experience, it is easy to identify the IHP plane. It runs medial to the internal iliac vessels as well as the vesicle pedicle with quite a small distance and then travels on the tip and lateral side of the SVs. Accordingly, we present some technical considerations with regard to IHP protection after exposure. First, due to the proximity to the IHP, excessive dissection and undue concentration should be avoided medial to the internal iliac artery. Second, we clip and transect the bladder pedicles adjacent to the internal iliac artery when there is sufficient exposure of its branches and avoid the internal pudendal artery injury. Third, the SVs should be followed to dissect them from their medial side, where almost no autonomic nerves exist, to the lateral surfaces.
After protecting the IHP, the next crucial step is NVB manipulation in the prostate area. Any surgical technique that minimizes injury to the NVBs in the prostate is always preferable [19] as nerve fibers around the prostate are densest in the posterolateral region, with approximately 20% of the fibers being anterolateral [20]. Maximal preservation of the peripheral nerves of the prostate is beneficial to postoperative functional recovery – and from an anatomic point of view, maintaining the integrity of the fasciae around the prostate as much as possible would help preserve these nerves. This is why we also strive to preserve the DVF in place without an incision. In our protocol, we dissected the ventral aspect of the DVF close to the posterior prostate capsule and created a plane toward the apex with proper DVF countertraction (Fig. 2b). The nerves that are sparsely located posterior to the prostate might be preserved through this procedure, and these nerves also contribute to erectile function [21].
Erectile function outcomes represent the most concerning target of nerve-sparing RC. Hekal et al. [22] reported an excellent potency rate of 78.8% with an average patient age of 48 years. In the Karolinska Institute study, satisfactory sexual function after 12 months was achieved in approximately 63% of patients [23]. In our cohort, 10 of 11 patients (90.9%) recovered normal erections within 1 year with or without sildenafil; this is comparable to the currently available results. Our approach differs from others in the initial precise dissection and protection of the IHP and shows promising functional results of clinical significance.
Preserving the nerves and related pelvic structures is conducive to maintaining continence [24, 25]. With continence being defined as being pad free or using a security liner, a recent review showed that the daytime continence rate was between 83 and 100% and was 66–76% for nighttime [26]. In our recent follow-up using an identical definition, comparable results were seen with continence rates of 90.9% during the day and 72.7% at night.
Our research, however, has some limitations. The limited number of patients and insufficient follow-up duration make it difficult for us to draw definitive oncologic conclusions, even though we have observed no tumor recurrence thus far. Furthermore, this was a retrospective study conducted at a single center. Finally, our intraoperative observations and anatomic explanation that the IHP can be precisely dissected in the medial plane of the distal ureter must still be corroborated by others. Studies involving larger sample sizes and longer follow-up durations are therefore necessary.
Conclusions
Our investigation on the precise dissection of the IHP while protecting the NVB is a systematic project designed to ensure the integrity of the nerve trunk that stimulates erections. We present herein our stepwise technique for nerve-sparing RC, which could be of clinical significance.
Acknowledgments
The authors are grateful to Chang Liu, who is an outstanding medical illustrator, the entire staff of the Department of Urology, and the First Affiliated Hospital of Jinan University, and we thank AJE (www.aje.com) for its linguistic assistance during the preparation of the manuscript.
Statement of Ethics
This study was approved by the Ethics Committee of Jinan University (KYk-2021-027). Written informed consent was obtained from each patient.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Funding Sources
The study was supported by the Science and Technology Project of Yangjiang City (No. 2018126 to Dr. Lai) and the Leading Specialist Construction Project-Department of Urology, the First Affiliated Hospital, Jinan University (No. 711006).
Author Contributions
Peifeng Zhong: manuscript writing, acquisition of data, analysis, and interpretation of the data. Guohao Wu: manuscript writing and supervision. Haomin Li and Xianguo Hu: assistance with data collection. Bingquan Wu, Zexiong Guo and Yu min Zhuo: manuscript review. Xuesong Li and Caiyong Lai: project development, management, operations, and supervision.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Additional information
Peifeng Zhong and Guohao Wu contributed equally to this work.