Abstract
Introduction: The aim of the study was to examine cancer-specific mortality (CSM) of unconventional urethral cancers. Methods: Within the SEER (2004–2016) database, we analyzed CSM of 165 patients with unconventional urethral-cancer histology. Kaplan-Meier plots were used to test the effect of unconventional histologies in urethral cancer on CSM. Results: Of 165 eligible patients, the Mullerian type accounted for 55 (33.3%) versus melanocytic (26.7%) versus neuroendocrine 25 (15.2%) versus lymphoma 22 (13.3%) versus mesenchymal/sarcoma 15 (9.1%) versus spindle cell 4 (2.1%) patients. Median age at diagnosis was 81 years in spindle cell, 75 in melanocytic, 74 in neuroendocrine and mesenchymal/sarcoma, 67 in lymphoma, and 62 years Mullerian type (p < 0.001). Of all, 116 (70.3%) were female. The Mullerian type exhibited the highest female ratio (96.4%) versus the lowest female ratio in neuroendocrine (24.0%). The Mullerian type was most frequent in African-American females. In Caucasian females, the melanocytic type was most frequent (49.1%). In African-American (38.9%) and Caucasian males (33.3%), neuroendocrine histology was most frequent. Three-year CSM was, respectively, 27.5%, 23.1% 22.3%, 20.5%, and 16.1% for melanocytic, mesenchymal/sarcoma, Mullerian type, neuroendocrine, and lymphoma histology. Median cancer-specific survival was 106 versus 10 months for combined nonmetastatic versus metastatic nonconventional histologies. Conclusion: Important age, sex, racial/ethnic group distribution, and survival differences exist between each unconventional urethral-cancer histological subtypes.
Introduction
Primary urethral cancer is a very rare cancer with an incidence of 2.7 and 0.6 per million for men and women, respectively [1, 2]. Most common urethral-cancer histological subtypes are urothelial, squamous-cell carcinoma (SCC), and adenocarcinoma [3‒10]. Since urethral cancer has been studied in very small patient groups, only more historical studies as well as case reports have focused on other unconventional histological subtypes, instead of urothelial, SCC, and adenocarcinoma of the urethra [11‒20]. To the best of our knowledge, no previous investigation compared different unconventional urethral-cancer histologies.
We addressed this void and relied on the Surveillance, Epidemiology, and End Results (SEER) database (2004–2016). We hypothesized that patients’ sex and racial/ethnic distribution, as well as cancer-specific mortality (CSM) across unconventional histologies of urethral cancer may differ.
Material and Methods
Study Population
The current SEER database samples over 34% of the US population and approximates it in demographic composition and cancer incidence [21]. Within the SEER database 2004–2016, we identified patients aged ≥18 years with histologically confirmed urethral cancer (International Classification of Disease for Oncology site code C68.0). Histological subtypes were defined according to WHO criteria [22]. Urothelial, SCC, adenocarcinoma, and unknown histologies were excluded. Cases identified only at autopsy or death certificate were also excluded. TNM stage was used according the 8th edition of malignant tumors [23]. Racial/ethnic groups were defined as Caucasian, African-American (AA), Hispanic, or other racial/ethnic groups. Those selection criteria resulted in 165 urethral-cancer patients with unconventional histologies.
Statistical Analysis
Descriptive statistics included frequencies and proportions for categorical variables. Means, medians, and interquartile ranges were reported for continuously coded variables. The χ2 tested the statistical significance in proportions’ differences. The t test and Kruskal-Wallis test examined the statistical significance of means’ and distributions’ differences. Trend tests were performed to explore differences and according to stage at presentation.
Descriptive characteristics were tabulated. Subsequently, Kaplan-Meier plots and multivariable cox regression models were fitted to depict CSM for unconventional histological urethral-cancer subtypes, as well as in the overall cohort of metastatic urethral cancer. All tests were two sided with a level of significance set at p < 0.05 and R software environment for statistical computing and graphics (version 3.4.3) was used for all analyses.
Results
Descriptive Characteristics of the Study Population
Of 165 eligible urethral-cancer patients, the Mullerian type accounted for 55 (33.3%) versus melanocytic type (26.7%) versus 25 (15.2%) neuroendocrine (NEC) versus 22 (13.3%) lymphoma versus 15 (9.1%) mesenchymal/sarcoma versus four (2.1%) spindle cell patients, respectively (Table 1). Of all unconventional urethral-cancer patients, 116 (70.3%) were female. Median age at diagnosis was highest in spindle cell urethral cancer (81 years), followed by melanocytic type (75 years), NEC and mesenchymal/sarcoma (both 74 years), lymphoma (67 years), and Mullerian type (62 years), in that order (p < 0.001). Overall, 20 patients (12.1%) harbored metastatic disease, of whom 9 (45.0%) received chemotherapy. Median follow-up was 19 months (IQR 9–48) and was longest in the Mullerian type (28 months, IQR 17–51) and shortest in spindle cell carcinoma (2 months, IQR 1–2). Finally, also secondary urethral-cancer rates differed according to unconventional urethral histology and ranged from 9.1% (Mullerian type) to 73.3% mesenchymal/sarcoma.
Sex Distribution in Unconventional Histologies or Urethral Cancer
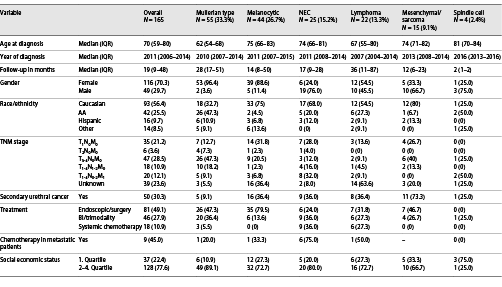
Overall, females accounted for 70.3% of all patients with unconventional histology of urethral cancer (Fig. 1). Moreover, absolute rates of females differed significantly across all unconventional urethral-cancer histologies (p < 0.01). The lowest proportion of females was recorded in NEC histology (24.0%) and spindle cell (25.0%) as well as mesenchymal/sarcoma tumors of the urethra (33.3%). Conversely, the highest rates of female patients with unconventional histologies of urethral cancer were recorded in melanocytic (88.6%) and Mullerian-type histology of urethral cancer (96.4%). Almost equally distributed sex proportions were recorded in lymphoma histology (54.5% females).
Stacked barplots depicting sex distribution according to unconventional histological subtypes of 165 urethral-cancer patients. NEC, neuroendocrine; Spindle, spindle cell.
Stacked barplots depicting sex distribution according to unconventional histological subtypes of 165 urethral-cancer patients. NEC, neuroendocrine; Spindle, spindle cell.
Distribution of Unconventional Histologies of Urethral Cancers in Racial/Ethnic Groups according to Patient Sex
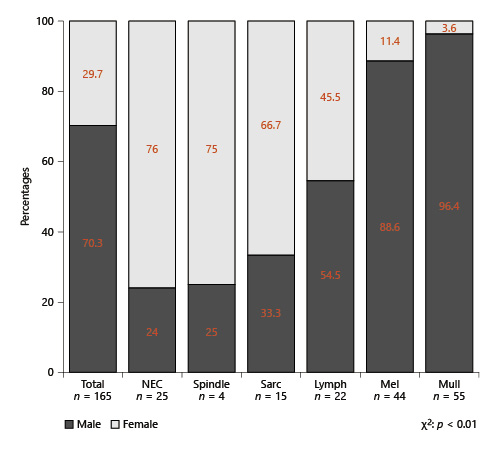
Mullerian-type urethral cancer (Fig. 2) was most frequent in AA females (75.8%), followed by Hispanics (50%), other racial/ethnic groups (35.7%), and Caucasians (29.8%), in that order. Conversely, the melanocytic type was most frequently in Caucasian females (49.1%) and 42.9% in other racial groups, 25% in Hispanics, and 6.1% in AA females with unconventional urethral cancer. In AAs (38.9%), as well as Caucasian males (33.3%), NEC histology was the most frequent unconventional histology of urethral cancer. Conversely, in males, Mullerian-type tumor accounted only for 11.1% in AAs and 2.8% in Caucasians. Due to few observations, comparisons could only be made between Caucasians and AAs in males.
Stacked barplots depicting the distribution of unconventional urethral-cancer histological subtypes according to racial/ethnic groups in females (a) and in males (b). Due to few observations, no analyses could be performed according to Hispanics and other racial/ethnical groups in males. NEC, neuroendocrine; AA, African-American.
Stacked barplots depicting the distribution of unconventional urethral-cancer histological subtypes according to racial/ethnic groups in females (a) and in males (b). Due to few observations, no analyses could be performed according to Hispanics and other racial/ethnical groups in males. NEC, neuroendocrine; AA, African-American.
CSM Differences in Unconventional Histologies in Nonmetastatic and Metastatic Urethral Cancer
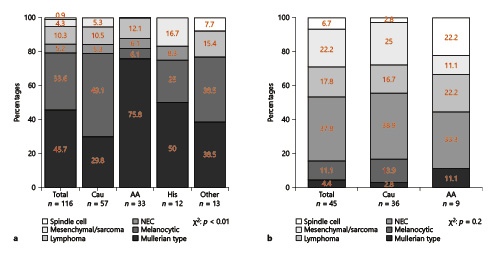
Due to heterogeneity in natural tumor histology, we first focused on CSM differences in nonmetastatic unconventional histologies of urethral cancer. Here (Fig. 3), 3-year CSM was, respectively, 27.5%, 23.1% 22.3%, 20.5%, and 16.1% for melanocytic, mesenchymal/sarcoma, Mullerian type, NEC, and lymphoma histology of urethral cancer. In Mullerian-type tumor, median cancer-specific survival was 53 months. Due to few observations, no CSM curve for spindle cell histologies could be computed.
Kaplan-Meier plot illustrating cancer-specific mortality (CSM) for nonmetastatic urethral-cancer patients according to unconventional histological subtypes. NEC, neuroendocrine.
Kaplan-Meier plot illustrating cancer-specific mortality (CSM) for nonmetastatic urethral-cancer patients according to unconventional histological subtypes. NEC, neuroendocrine.
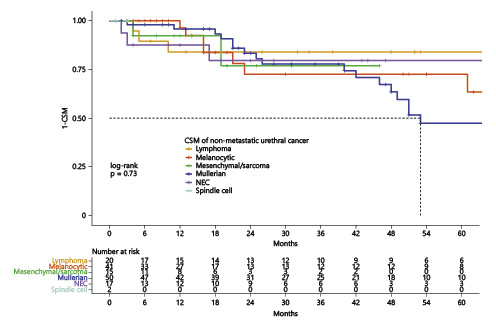
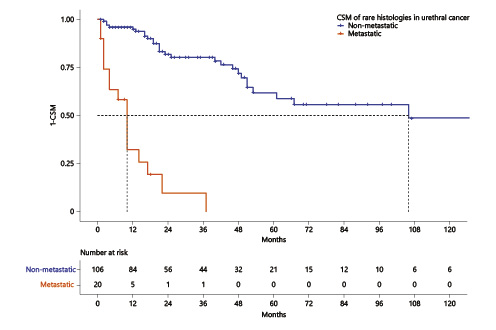
After combining unconventional histologies of nonmetastatic urethral cancer, median cancer-specific survival was 106 months (Fig. 4). Conversely, median cancer-specific survival of unconventional histologies of metastatic urethral cancer was 10 months.
Kaplan-Meier plots illustrating cancer-specific mortality (CSM) for nonmetastatic (a) and metastatic (b) unconventional urethral-cancer histological subtypes.
Kaplan-Meier plots illustrating cancer-specific mortality (CSM) for nonmetastatic (a) and metastatic (b) unconventional urethral-cancer histological subtypes.
Discussion
We hypothesized that patients’ sex and racial/ethnic distribution, as well as cancer-specific survival across unconventional histologies of urethral cancer may differ. We tested this hypothesis within the SEER database and made several important observations.
First, we identified important differences in baseline characteristics of unconventional histologies of urethral cancer. Specifically, Mullerian-type tumor was the most frequent (33%) melanocytic cancer (27%), the second most frequent histology of unconventional urethral-cancer histologies. Conversely, spindle cell histology is a very rare histological subtype (2.4%) of urethral cancer. Moreover, median age at diagnosis was youngest in Mullerian-type tumor (62 years), and the oldest age at diagnosis was recorded in spindle cell histology (81 years). To the best of our knowledge, no previous investigation focused comparisons of unconventional urethral-cancer histologies. In consequence, our data cannot be directly compared to other studies since those do not exist. However, a more historical population-based study by Patel et al. [11] (SEER database 1973–2014) also investigated the epidemiology of 61 patients with Mullerian-type histology of urethral cancer. In consequence, Mullerian-type histology may represent the fourth most common histology in urethral cancer after urothelial, SCC, and adenocarcinoma [2, 3, 10, 24, 25]. Since the literature provides only case reports about melanocytic tumors of the urethra and bladder cancer, the largest cohort of melanocytic tumors consists of 16 patients; our study provides the largest contribution to the medical literature of the epidemiology of those unconventional urethral-cancer histological subtypes [26, 27].
Second, we identified important differences in unconventional histological subtypes of urethral cancer according to patients’ sex. In total, females exhibited more frequently unconventional histologies of urethral cancer than males (70 vs. 30%). Moreover, the highest proportion of males was recorded in NEC (76%) and spindle cell histology (75%). Conversely, the highest proportions of females were recorded in the predominant histological subtype of unconventional histologies, namely, Mullerian-type (96%) or melanocytic histology (89%). These observations indicate a higher proportion of females harboring rare histologies of urethral cancer and are in an agreement with previous publications [4, 5, 12, 24, 28]. For example, Abudurexiti et al. [12] also showed higher proportions of females with nonurothelial, non-SCC, and nonadenocarcinoma histologies of urethral cancer, relative to the three most frequent histological subtypes, where males are usually predominant except for adenocarcinoma histological subtype.
Third, in further analyses, we stratified the patients’ sex distribution according to four examined racial/ethnic groups regarding unconventional urethral-cancer histologies. Here, in Caucasian and other racial/ethnic group females, the most frequent rare histology was melanocytic tumor (49 and 43%). Conversely, in AA and Hispanic females, the most frequent unconventional histology was the Mullerian type (76 and 50%). Conversely, in Caucasian and AA males, the predominant unconventional histology of urethral cancer was NEC tumors (39 and 33%). These observations are important since incidence rates of urethral-cancer patients differ between sexes and additionally also differ between race/ethnic groups as it was already shown for example in adenocarcinoma urethral cancer in AA females [1, 29].
Finally, CSM analyses of nonmetastatic and metastatic unconventional urethral-cancer histologies yielded important observations. For example, no clinically meaningful CSM differences in nonmetastatic unconventional urethral-cancer histology patients could be observed within the first 2 years after diagnosis. Unfortunately, due to few observations, no separate CSM analyses could be computed for males and females.
Additionally, nonmetastatic unconventional urethral-cancer histology patients harbored a median survival of 106 months. Conversely, metastatic unconventional urethral-cancer histology patients harbored a median survival of 10 months. These data may show a survival improvement relative to the more historical study by Abudurexiti et al. [12] (1978–2015) with a median CSM of 61 months for rare urethral histologies in general, without stratifying according to metastatic versus nonmetastatic disease. These observations should be considered in clinical practice, when patients with unconventional histologies of urethral cancer are seen and counseled.
Our work has limitations and has to be interpreted in the context of its retrospective and population-based design. Our cohort is based on a relatively small sample size that resulted in lack of significant differences in some subgroup comparisons. However, it should be emphasized that the SEER database is designed with the intent of providing proportional representation of the US population. In consequence, few if any other databases will provide a larger sample of those excessively rare unconventional histological subgroups of urethral-cancer patients. As in all SEER-based analyses, comorbidities were not available and could lead to confounding of CSM rates. Finally, due to small sample sizes, we grouped patients within unconventional urethral-cancer histologies, as previously reported for unconventional histologies [30]. As a result, it is possible that some of these individual subtypes of unconventional histologies may have more favorable survival than others or vice versa (e.g., in lymphomas). In consequence, specific conclusions regarding comparisons of CSM between individual subtypes of unconventional histologies need to be made with caution. Moreover, differences in primary and secondary cancer of the urethral cancer may exist [31].
Conclusion
Taken together, unconventional urethral-cancer histologies are excessively rare, and nonetheless, differences exist between each unconventional histological subtype according to age at diagnosis, sex distribution, as well as distribution according to racial/ethnic groups. Second, Mullerian-type tumor is the most frequent unconventional histology of urethral cancer, followed by melanocytic-type histology. Finally, CSM differences in nonmetastatic unconventional histologies are marginal, and survival in metastatic stage is dismal.
Statement of Ethics
Ethical approval was not required for this study in accordance with local/national guidelines. Written informed consent from participants was not required in accordance with local/national guidelines.
Conflicts of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This manuscript did not receive any funding.
Authors Contributions
Wenzel Mike: manuscript writing/editing, protocol/project development, and data analysis. Collà Ruvolo Claudia, Kluth Luis, Chun Felix K. H, and Karakiewicz Pierre: manuscript writing/editing and protocol/project development. Würnschimmel Christoph: data collection and management. Nocera Luigi, Hoeh Benedikt, and Tian Zhe: data analysis. Saad Fred, Briganti Alberto, Tilki Derya, Banek Severine, Mandel Philipp, and Becker Andreas: manuscript writing/editing.
Data Availability Statement
Data for this manuscript are derived from the SEER database, which can be found online at https://seer.cancer.gov.