Abstract
Purpose: The aim of the study was to assess quality of life (QoL), decision involvement, and decisional regret after treatment with vascular-targeted photodynamic therapy (VTP) (TOOKAD®) for unilateral low-risk prostate cancer. Methods: Validated questionnaires (EORTC QLQ-C30 and QLQ-PR25) capturing QoL post-treatment, involvement in decision-making (Control Preferences Scale) and decision regret (Decisional Regret Scale), were given to patients at the 12-month visit after undergoing VTP at our institution between May 2018 and February 2021. Results: Out of 44 patients, 36 patients were included in this study and 31 (86.1%) responded to the questionnaires. Mean overall health score capturing QoL at 12 months was 79.3 (standard deviation: ±18.1). 70.9% of the patients (n = 22) had no decision regret, and 67.8% of men (n = 21) had an active role in decision-making. In control biopsy at 12 months post-treatment, 19.4% of patients (n = 7) presented with local recurrence and progression to higher Gleason score (GS) was found in 13.8% of patients (n = 5). Patients (n = 3) presenting with tumor recurrence or progression to higher GS in control biopsy showed a significantly higher level of decision regret (p < 0.009). Conclusion: Only 9.7% of men (n = 3) felt a strong emotion of regret at 12 months after VTP. Level of decision regret was significantly higher in patients with local recurrence or tumor progression detected in control biopsy. QoL was stable after VTP.
Introduction
Vascular-targeted photodynamic therapy (VTP) with the photosensitizer padeliporfin (TOOKAD® soluble) is a new focal therapy (FT) approach for treatment of unilateral low-risk prostate cancer (PCa) offering an alternative to active surveillance (AS) [1-3]. The randomized PCM 301 trial showed that VTP significantly reduced the incidence of aggressive PCa in the follow-up after VTP. Consequently, fewer patients underwent radical treatment [1]. Whereas classical curative procedures such as radical prostatectomy (RP) and radiotherapy might be associated with a significant loss of bladder and sexual function, the hallmark of FT is sufficient oncological outcome combined with low appearance of morbidities [4-6]. However, due to its novelty long-term oncological outcomes after VTP are still rare. Men with diagnosed PCa are required to make a difficult decision regarding to their primary treatment, which is particularly challenging due to the very different available therapeutic approaches.
Regret to a medical decision, which is a negatively experienced emotion and reduced quality of life (QoL) in men with PCa, is associated with how the decision with regard to the treatment has been made as well as with the final oncological results of the decision [7]. Earlier studies for men undergoing surgery for PCa showed that more active participation in decision-making as well as the collaboration between patients and their physicians is associated with higher QoL and less regret with treatment [8, 9].
Decision regret and QoL after RP or radiotherapy have been investigated in many previous studies; however, there are no data on QoL and decision regret for patients treated with VTP [10, 11]. Evaluation of decision regret and QoL is of major importance, since FT stands for preservation of sexual function and continence in patients with focal low- and intermediate-risk PCa [12, 13]. Therefore, our aim was to better understand men’s decision-making for PCa treatment with VTP and to investigate decision regret and QoL in this group of patients.
Patients and Methods
Patient Selection and Questionnaires
Forty-four consecutive patients receiving VTP for unilateral low-risk PCa at our institution between May 2018 and February 2021 were included. Inclusion criteria were a unilateral low-risk PCa of clinical stage cT2a, Gleason score (GS) ≤6, and PSA ≤10 ng/mL. In pretreatment prostate biopsy, a maximum of 3 positive cores with a maximum tumor length of 5 mm/core, or 1 to 2 positive cores with tumor infiltration of ≥50% per core, or PSA density ≥0.15 ng/mL2 was allowed.
Validated questionnaires (EORTC QLQ-C30 and EORTC QLQ-PR2) were sent by post or handed to patients during clinical follow-up visits at 12 months after the initial therapy. Oncological follow-up after VTP consisted of regular 3 monthly PSA monitoring. At 9–12 months post-treatment, multiparametric MRI and targeted biopsy in case of evidence of PI-RADS ≥3 lesions, and a systematic biopsy covering the treated (infield) and untreated (outfield) lobes were performed.
In total, 36 patients were eligible for participation in the present study. One patient was excluded due to receiving VTP on the other lobe due to tumor recurrence. Three patients were excluded due to insufficient follow-up time. Another 3 patients were lost to follow up, and 1 patient died of a PCa unrelated cause.
We used the Decisional Regret Scale (DRS) at the 12-month visit to measure distress after the decision to have VTP for low-risk PCa. DRS consists of 5 questions, with a Likert-type response (1: “strongly agree”; 2: “agree”; 3: “neither agree nor disagree”; 4: “disagree”; and 5: “strongly disagree”). Questions 2 and 4 are reverse-coded to prevent response yea-saying bias [14]. For calculation of the final score with a range between 0 and 100, the 2 negatively phrased questions were reversed. Each scale was subtracted by 1, and then, the sum of the 5 items was multiplied by 25. A score of zero meant no regret, and a score of 100 indicated a high level of regret. Scoring was further categorized into high (>65), medium (>25–65), or low (<25) levels of regret.
A Cronbach’s α coefficient of 0.83 to measure internal consistency for DRS was previously calculated for patients receiving RP [8]. To investigate patient’s involvement in treatment decision, we used the validated Control Preferences Scale (CPS), which was administered to patients at the 12-month follow-up visit. CPS was developed by Degner et al. [15] and consists of 5 statements regarding the role of patients in decision-making for their treatment.
The possible responses were as follows: 1 “patient makes the decision alone”; 2 “patient makes decision after consideration of physician opinion”; 3 “patient and physician make the decision together”; 4 “physician makes the decision with input from patient”; 5 “physician makes the decision without input from patient.”. For our analysis, we categorized statements 1 and 2 into “active role,” statement 3 into “collaborative role,” and statements 4 and 5 into “passive role.”
QoL was assessed by European Organisation for Research and Treatment of Cancer (EORTC) QoL questionnaires EORTC QLQ-C30 and EORTC QLQ-PR25. The Quality of Life Questionnaire Core 30 consists of 30 questions and uses a self-reported Likert scale (1–7 for the overall health status and 1–4 for all other items). This questionnaire evaluates global health-related QoL, 5 functional scales (physical, emotional, role, cognitive, and social), and 9 symptoms (e.g., fatigue, pain, etc.).
The Quality of Life Questionnaire PCa-specific module (QLQ-PR25) consists of 25 items with a Likert-type scale response (from 1 to 4) with 1: “not at all”; 2: “a little”; 3: “quite a bit”; and 4: “very much.” It measures 9 urinary functional symptoms (e.g., dysuria, incontinence, etc. [PRURI, PRAID]). Four items assess bowel function (PRBOW), 6 items measure treatment-related symptoms (PRHTR), and 6 questions are related to sexuality (PRSAC, PRSFU).
We interpreted both QoL questionnaires according to the EORTC guidelines. The system of measurement for both QoL questionnaires presents a score of 0–100. A higher score of the scales related to functions is associated with a better QoL. A higher score of the scales for the assessment of symptoms represents a worse level of symptoms.
Statistical Analysis
Data analyses of quantitative variables were presented as numbers (n), median, mean, standard deviation (SD), minimum and maximum, and range. We applied linear regression models for oncological (e.g., progression, recurrence), social (e.g., level of education, marital status, and role in decision-making) as well as clinical (e.g., age) variables to predict different levels of decisional regret. Further, general global health status and QoL scores were entered into regression models to measure the level of decision regret. A p value of <0.05 was defined to indicate statistical significance. Statistical analysis was performed using the SPSS 27.0 (IBM corp., Armonk, NY, USA) software.
Results
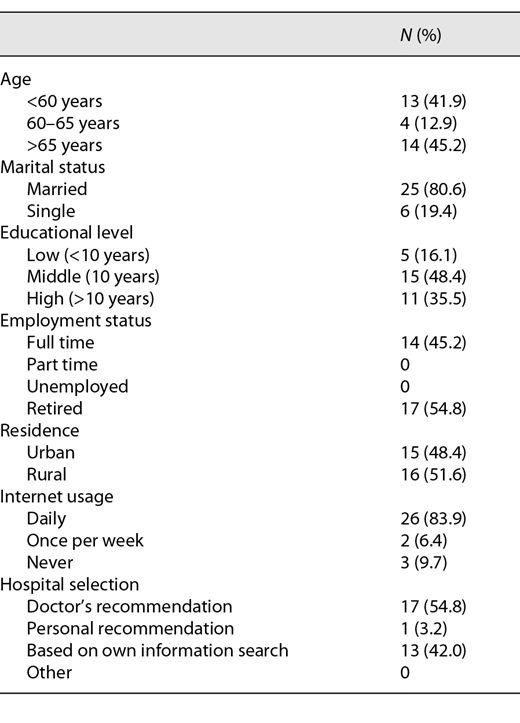
Thirty-six patients who underwent VTP at our institution were eligible for participation. The response rate was 86% (n = 31). The median time from VTP to the date of investigation was 19 months (range: 1–33 months). Patient demographics are shown in Table 1. The median age was 63 years (range: 57–71 years). 35.5% of the men (n = 11) were university educated, 45.2% (n = 14) were employed full time, and 80.6% (n = 25) were married. 83.9% of patients (n = 26) used the Internet on a daily basis for information research including health-related topics. 54.8% of the patients (n = 17) followed their doctor’s recommendation on hospital selection.
The DRS is depicted in Table 2. 70.9% of patients (n = 22) had no decision regret. Three patients (9.7%) had a clinical significant level of decision regret (score >25). The mean score of the DRS was 7.6 (SD: 21.4).
(a) Distribution of DRS answers of 31 patients after VTP therapy (n [%]) and (b) patients’ involvement in decision-making (CPS)
![(a) Distribution of DRS answers of 31 patients after VTP therapy (n [%]) and (b) patients’ involvement in decision-making (CPS)](https://karger.silverchair-cdn.com/karger/content_public/journal/uin/106/9/10.1159_000520084/1/m_000520084_t02.png?Expires=1772316444&Signature=p3RvgBfFqqipZtwoZ2xL-H6jiWB57Akvw8tw8N4TpiFq8gWIkcz2ED7TBjDvFtvu~bMKrd6yvzuEwXTnLagIOUOC8qF1DUqId2X9RuDcCccSxbbIuKsyEkgvCfS66nUYFz5cQbh7ZnxExlCMBfrMGJdlr-KcCxNBB03jbvOhCt2YM4eLV~KouSD7YYHxA3RYTVclb4xv8cqnanTyGZtt7UbP342c08gu5tP5ZkWvo-ZCn8TuDQEM~aGHhXtVhwNWYLLttn52bX4KfXU~T4KObHzbtajrTtL8AuRLxh13ChhrZ~Gs8k~gtIWRXJWGnoFdwNoKbm8ITgNMmIr-a7-Qtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
In the sub-analysis, patients (n = 3) presenting with tumor recurrence or progression to higher GS in control biopsy at 12 months after VTP showed a significantly higher level of decision regret (p < 0.009). Two of the 3 patients with significant level of decision regret had a progression to GS ≥7 and 1 patient presented with a GS 6 tumor recurrence. All 3 patients received definitive treatment with 2 of them undergoing RP and 1 patient undergoing radiation therapy. Age (p = 0.125), marital status (p = 0.269), or level of education (p = 0.440) did not show an association on decision regret.
Furthermore, role of decision involvement had no impact on the level of regret after FT with VTP (p = 0.136). Patients’ involvement in decision-making regarding treatment for PCa is shown in Table 2.
67.7% of our patients (n = 21) had an active role in decision-making. Most of the patients decided on VTP by themselves (n = 7) or with their physicians together (n = 23).
The mean functional and symptom scores after VTP treatment for the EORTC QLQ-C30 and QLQ-PR25 are displayed in Table 3. QoL at 1-year post-treatment remained on a high level with a mean QLQ-C30 overall health status of 79.3% (SD: 18.1). Patients with higher overall health status had significantly lower level of decision regret (p = 0.032). Mean urinary symptoms after VTP were low at 18.0 (SD: 16.1). Sexual function was stable with a mean of 62.1 (SD: 17.4).
Discussion
FT is gaining popularity as an established alternative to AS and to radical therapy options for treatment of localized low- and intermediate-risk PCa [16]. Hence, it is important to investigate and display patients’ perception of their QoL, role of involvement in decision-making as well as their level of decision regret after focal treatment for PCa.
The present study was performed to evaluate those patient-related outcomes after treatment with VTP for PCa. Previously, we had reported our results on short-term oncological and functional outcomes after VTP [3]. We were able to show a low complication profile as well as a good functional outcome after VTP. However, in 12- and 24-month control biopsy after VTP, disease recurrence as low- and intermediate-risk PCa was detected in 27% of patients [3].
In the present study, only 9 patients stated some level of decisional regret regarding their VTP treatment. 19.4% of those patients (n = 6) showed low level of regret. Clinically relevant level of decision regret (score >25) was seen in 9.7% of patients (n = 3). We assume that the high level of regret in these patients is related to local tumor recurrence/progression in control biopsy. Consequently, 2 of them underwent RP and 1 patient received external beam radiation therapy. Westhoff et al. [17] investigated decision regret among patients receiving FT with high-intensity focused ultrasound (HIFU) for low- and intermediate-risk PCa. Similar to our results, the authors showed overall low rates of unsatisfied patients. They reported that overall 20.8% of patients had regret regarding their treatment decision [17].
Decisional regret after RP or radiotherapy for PCa was investigated in multiple studies [18, 19]. Diefenbach et al. [20] were able to show that sexual and urinary dysfunctions were strong predictors for treatment-related regret in their analysis. Interestingly, lower levels of regret were seen in patients opting for external beam radiation or brachytherapy. Thus, we assume that low levels of regret in our patients are partly related to good functional outcomes and to a lesser extent to sufficient short-term oncological outcomes after VTP. Repetto et al. [21] investigated regret in patients, which followed an active AS protocol. The results showed that most patients had low level of regrets even after discontinuation of AS and receiving an active treatment [21].
Furthermore, several studies have described a higher level of decisional regret among patients, which were not actively involved in final decision-making [8]. van Stam et al. [8] described in a multicenter observational study that 17% of patients with localized PCa had less involvement in treatment decision than preferred. Consequently, these patients were associated with stronger decision conflict and decision regret [8]. In our analysis, all patients with decision regret were actively involved in decision-making and decided in concordance with their treating urologist on VTP treatment. Overall, more than 65% of patients in our cohort were actively involved in decision-making regarding their treatment approach. Baunacke et al. [9] showed that patients receiving robot-assisted radical prostatectomy (RARP) had a more active role in decision-making and used the Internet more frequently than in patients receiving open RP. The authors confirmed that functional and oncological outcomes were predictive of low decision regret. 87% of patients who underwent RARP used the Internet for health care-related topics [9]. We observed comparable results among our patients (84% used the Internet daily). Thus, patients opting for FT or RARP seem to perform deeper research regarding newer treatment modalities for PCa and are actively involved. Hilger et al. [22] showed that many men with localized PCa use the Internet for information and that physicians can reduce disease-specific anxiety by advising their patients about reliable online sources. Possible factors influencing the high level of involvement in our sample could be the novelty of the treatment, the unique focal approach, or a high level of education among the included patients. However, our analysis showed no significant association between the level of patient’s involvement in decision making and the level of decision regret. Marital status and level of education were not associated with decision regret in our analysis.
Davison et al. [10] were able to show that collaboration between the treating doctor and patient as well as shared decision-making for treatment was associated with a lower level of regret. Furthermore, active involvement in decision-making was associated with a higher QoL post-treatment. A study from 2013 investigating treatment regret and QoL following RP showed that reducing regret may even improve mental health in patients [23].
We evaluated QoL by using the EORTC QLQ-C30 and EORTC QLQ-PR25 questionnaires. The mean global health status in all our patients was high at 79.3. Comparable results were described by Hatiboglu et al. [24] in a single institutional study investigating QoL as well as functional and oncological outcome after HIFU treatment for PCa. Overall global health status after HIFU was slightly lower at 69.4 than in our analysis. The authors discussed that Global Health Score for the general German population, which was described by Schwarz et al. [25] at a score of 65.6 for patients <70 years and a score of 61.5 for patients >70 years. Thus, our patients were present with a considerable higher global health score even after treatment than in the general population. This could be partly explained by the fact that our included patients were actively concerned about their health status and therefore choose VTP treatment for PCa. Reported urinary symptoms and bother were low in our study population, which seems to be an advantage of FT in general. Sexual functioning and activity remained stable at 62.1 and 50.5, respectively. Umbehr et al. [26] investigated sexual activity and functioning as well as urinary symptoms and bowel symptoms after RP using EORTC QLQ-PR25. Results were considerably lower than in our cohort.
This study has several limitations that should be noted. Our single-center sample consists of a relatively small patient size. Furthermore, data were collected retrospectively. Follow-up window was considerably short, and questionnaires were only distributed post-treatment for most patients. Hence, an adequate comparison of changes in QoL before and after VTP treatment was not performed. However, since no data or other study investigating this topic is available at this point, these biases are acceptable. We are planning to present further results in the future.
Conclusion
Our analysis showed that 1 of 4 patients showed treatment-related regret at 12 months after VTP, which was significant in 1 of 10 patients. In our series, level of decision regret was markedly increased in the presence of local recurrence or tumor progression detected in control biopsy at 12 months post-treatment. Role of involvement showed no influence on the level of regret. However, shared decision-making as well as efforts to improve patient’s information and knowledge about therapy and related risks may reduce decision regret in general. Specific for our patients undergoing VTP, a good counseling pre- and postoperatively about possible side effects and risks of FT is mandatory. QoL was stable after VTP. To examine the long-term effects of FT with VTP on QoL and decisional regret, a longitudinal follow-up of these patients is required.
Acknowledgments
We would like to thank all the participants. Part of this study was presented at the 73rd Annual Meeting of the German Urologists’ Association, Stuttgart, Germany, September 15–18, 2021.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Review Board of the Medical Faculty Carl Gustav Carus, Technical University of Dresden, approval number EK-259062020. The written informed consent of each participant was obtained. Our study was performed in accordance with the Declaration of Helsinki.
Conflict of Interest Statement
The authors made no disclosures.
Funding Sources
The authors have received no external funding.
Author Contributions
L.F. and A.B. contributed to study concept and design. L.F., B.B., R.P., and A.Z. involved in data collection. L.F., M.B., B.B., R.P., A.Z., and A.B. performed analysis and interpretation of data. L.F. and A.B. drafted the manuscript. S.P., J.H., A.B., and C.T. involved in critical revision of the manuscript for important intellectual content.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.