Abstract
Background: Effective follow-up after living kidney donation is important for maintaining the renal function of the donor. We investigated whether the estimated glomerular filtration rate (eGFR) and urinary protein and enzyme levels can provide important information regarding the state of the remaining kidney after donor nephrectomy. Methods: Seventy-five living donations were included (prospective/retrospective) in the study. The following parameters were measured up to 1 year after donor nephrectomy: serum creatinine and cystatin C as markers of the GFR; the high-molecular-weight urinary proteins as markers of glomerular injury; and the low-molecular-weight urinary proteins and urinary enzymes as markers of tubular function. Results: One year after kidney donation, the creatinine and cystatin C values were 1.38-fold increased than their initial values, while the eGFR was 32% lower. At that time, 38% of donors had a moderate or high risk of CKD progression. The biochemical urinary glomerular and tubular kidney markers examined showed different behaviors. After a transient increase, the glomerular proteins normalized. Conversely, the detection of low-molecular-weight urinary proteins and enzymes reflected mild tubular damage at the end of the study period. Conclusions: Our findings suggest that for the evaluation of mild tubular damage, low-molecular-weight marker proteins should be included in the urine diagnostic of a personalized living kidney donor follow-up.
Introduction
In 2019, approximately one-fourth of all kidney transplants were made possible by a living donation [1]. The long-term safeguarding of donor health is a unique challenge, as donor nephrectomy (DN) transforms the donor from a healthy person into a patient. It is generally assumed that DN is safe [2, 3], but there are increasing doubts [4-8]. An important criterion for assessing patient safety is the risk of disease in the remaining single kidney or being required to undergo dialysis. Most studies are limited to the determination of the GFR and total urinary protein as kidney function parameters. After DN, organ-specific monitoring of the remaining kidney is rare [9-11]. Therefore, determination of a urinary protein profile is necessary [12, 13]. High-molecular-weight proteins such as immunoglobulin G (IgG), albumin (Alb), and transferrin (Tf) are detected in urine when the glomerular barrier is damaged, and low-molecular-weight proteins such as α1-microglobulin (α1M), β2-micro-globulin (β2M), retinol-binding protein (RbP), and the urinary enzyme N-acetyl-β-D-glucosaminidase (NAG) are found when tubular dysfunction or injury occurs. We investigated changes in the glomerular and tubular function of the remaining single kidney after DN. Are differences present among the kidney compartments? What is the postoperative course of these urinary biomarkers up to 1 year after surgery?
Materials and Methods
Seventy-five consecutive living donor nephrectomies were included in the study: 33 open (retrospective) and 42 hand-assisted laparoscopic (prospective) donor nephrectomies. Our project was approved by the Local Ethics Committee of the Martin Luther University Medical School Halle and written informed consent of the living donors obtained (Reference No. 2016-163). All living donors fulfilled the inclusion criteria according to the Amsterdam Forum [14]. The selection criteria for open and hand-assisted laparoscopic surgery were identical. Furthermore, the type of surgery was dependent on surgeon preference. The Ethical Guidelines of Human Medical Research of the Helsinki Declaration were followed. The following parameters were determined: serum creatinine and cystatin C levels (S-Crea and S-Cys C); total protein (U-Prot); the high-molecular-weight urinary proteins IgG, Alb, and Tf; and the low-molecular-weight urinary proteins RbP, α1M, and β2M and the urinary enzyme NAG. Urinary α2-macroglobulin was examined as a marker for the presence of postrenal blood. Urine and serum samples were obtained preoperatively and at 1, 2, 3, and 4 days; 1, 3, and 6 months; and 1 year after surgery (T0–T8). To compensate for the concentration fluctuations of spot urine, the urinary markers were related to urinary creatinine. The urinary proteins and S-Cys C were determined nephelometrically (BN2; Siemens Healthcare Diagnostics GmbH, Eschborn, Germany), S-Crea was measured with the Jaffe method (SYNCHRON Lx-system; Beckmann Coulter GmbH, Krefeld, Germany), and NAG was determined colorimetrically using Cobas Mira (Roche, Mannheim, Germany). The eGFR was calculated using the cystatin C- and creatinine-based formula from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), CKD-EPIcys-creaeGFR [15]. The risk of CKD progression was determined according to the criteria defined by the NKF/KDOQI based on GFR categories (G) and albuminuria (A) [15]. The descriptive statistics were calculated for all parameters. The course after DN is graphically displayed. For all measured proteins, the differences between the medians at baseline and the single measurement time points were determined by Wilcoxon matched-pair signed rank tests. The percentage of patients with abnormal urinary protein patterns at 1 year after the live donation was determined. The patterns were classified as glomerular, tubular, or mixed proteinuria based on the available urinary protein values. p values <0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 6 (La Jolla, CA, USA) or SPSS 22 (IBM, Ehningen, Germany).
Results
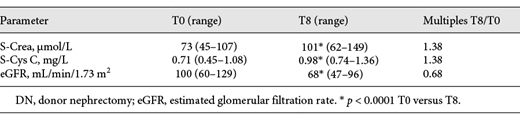
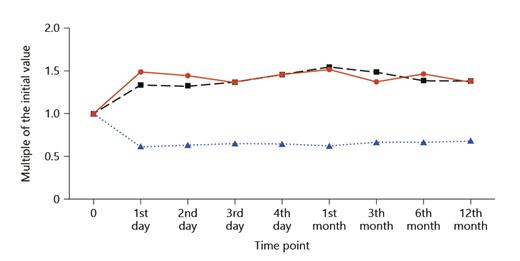
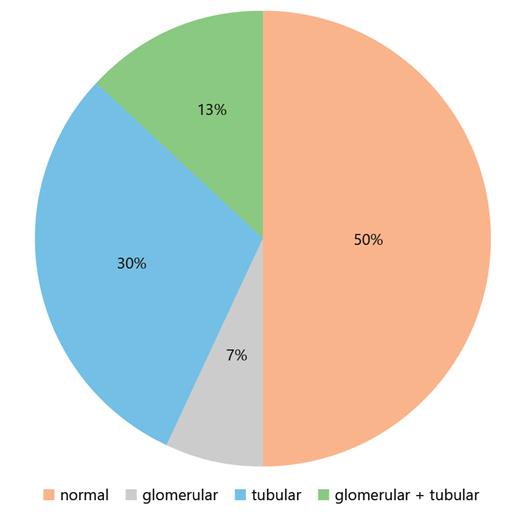
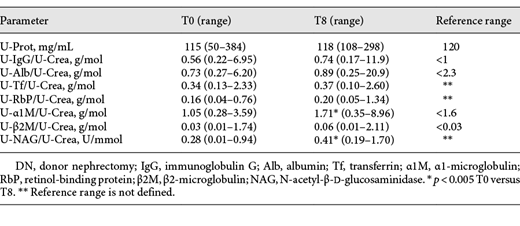
The patient characteristics are shown in Table 1. The GFR markers increased to a maximum level (S-Crea and S-Cys C) or declined to a minimum level (CKD-EPIcys-createGFR) within 1 month. Thereafter, renal function stabilized. After 1 year, the S-Crea and S-Cys C values were approximately 1.38 times greater than and the eGFR was 32% less than the preoperative value (Table 2; Fig. 1). According to the criteria defined by the NKF/KDOQI, 1 year after DN, 34% of living donors showed a moderate (G1A2, G2A2, and G3aA1) and 4% a high risk (G3aA2) of CKD progression [15] (G1, G2, and G3a correspond to GFR ≥90, 60–90, and 45–59 mL/min/1.73m2; A1 and A2 correspond to albuminuria <3 and 3–30 g/mol creatinine, respectively). We observed a transitory increase in all urinary proteins, reaching maximum values on the second or third day after surgery, but the increase was different for individual proteins. In contrast to the glomerular urinary proteins, the tubular proteins and the urinary enzyme NAG exhibited a greater increase (glomerular 1.83–3.97-fold and tubular 3.46–151-fold) and a slower decrease (Fig. 2). One year after DN, the mean and median values of all urinary proteins were greater than the preoperative values. The glomerular urinary proteins had declined into the reference range, and the tubular proteins α1M and β2M remained higher. At all time points (T1–T8), α1M and NAG were significantly increased compared to baseline (Table 3). Urinary α2-macroglobulin could not be detected at any time point; therefore, the results were not influenced by postrenal blood. One year after nephrectomy, only 50% of the live donors had a normal urinary protein pattern. The high proportion of pure tubular urinary patterns was surprising (30%; Fig. 3). Abnormal urinary protein patterns were not only found in patients at moderate or high risk of CKD progression but also in patients at low risk (37%).
Urinary proteins and NAG: median preoperative (T0) versus the median at 1 year after DN (T8)
S-Crea, S-Cys C, and eGFR after DN: multiples of the initial value versus measurement time points. d, day; mo, month; S-Crea, serum creatinine; S-Cys C, serum cystatin C; eGFR, glomerular filtration rate according to the CKD-EPIcys-creaeGFR formula; DN, donor nephrectomy.
S-Crea, S-Cys C, and eGFR after DN: multiples of the initial value versus measurement time points. d, day; mo, month; S-Crea, serum creatinine; S-Cys C, serum cystatin C; eGFR, glomerular filtration rate according to the CKD-EPIcys-creaeGFR formula; DN, donor nephrectomy.
Urinary proteins and NAG after DN: multiples of the initial value versus measurement time points. IgG, immunoglobulin G/U-Crea; Alb, albumin/U-Crea; Tf, transferrin/U-Crea; α1M, α1-microglobulin/U-Crea; RbP, retinol-binding protein/U-Crea; β2M, β2-micro-globulin/U-Crea; NAG, N-acetyl-β-D-glucosaminidase/U-Crea; U-Crea, urinary creatinine; DN, donor nephrectomy.
Urinary proteins and NAG after DN: multiples of the initial value versus measurement time points. IgG, immunoglobulin G/U-Crea; Alb, albumin/U-Crea; Tf, transferrin/U-Crea; α1M, α1-microglobulin/U-Crea; RbP, retinol-binding protein/U-Crea; β2M, β2-micro-globulin/U-Crea; NAG, N-acetyl-β-D-glucosaminidase/U-Crea; U-Crea, urinary creatinine; DN, donor nephrectomy.
Percentage distribution of urinary protein patterns at 1 year after living donation. Normal, no abnormalities; glomerular, at least 1 glomerular protein is increased; tubular, at least 1 tubular protein is increased; glomerular + tubular, at least 1 glomerular and 1 tubular protein are increased.
Percentage distribution of urinary protein patterns at 1 year after living donation. Normal, no abnormalities; glomerular, at least 1 glomerular protein is increased; tubular, at least 1 tubular protein is increased; glomerular + tubular, at least 1 glomerular and 1 tubular protein are increased.
Discussion
Patient Characteristics
Parents and life partners donate a kidney most frequently, with the high proportion of women. Fact is that women are more altruistic than men and see kidney donation as a duty to help their suffering child or partner [16]. The type of surgery has no influence on the donor kidney function in our study population, which was confirmed in the analysis of similar patient collective in our clinic (data not shown). Such results were also demonstrated in other trials [17, 18]. In the guideline originated form the Kidney Disease: Improving Global Outcomes (KDIGO) Executive Committee in consultation with the Transplantation Society, the “mini-open,” laparoscopy or hand-assisted laparoscopy by trained surgeons is suggested as the optimal approach to DN [19]. Additionally, in our investigation, we did not distinguish between left-sided and right-sided DN. The investigations of Weigand et al. [20] confirmed the correctness of our decision and summarized that also the right-sided DN is a safe procedure.
Markers of the Glomerular Filtration Rate
Markers of the GFR (S-Crea and S-Cys C) and the creatinine-based GFR are most frequently used for the assessment of renal function and for follow-up of living donors. Compared to S-Crea, S-Cys is more sensitive for detecting changes in renal function [21]. Due to the difficult method, the GFR is not generally measured, but formulas to calculate the eGFR, for example, based on S-Crea or S-Cys C levels, are used. The most suitable formula for follow-up of living donors has been debated [22-24]. The deterioration of the eGFR that occurs shortly after kidney donation (by up to 50%) and the subsequent stabilization of renal function approximately 1 month later conform to previously published data [25, 26]. Compensatory mechanisms in the remaining kidney are considered to account for the improvement of the eGFR over time [27]. One year after DN, renal function is reduced by approximately 30% of the preoperative value [28-30], which confirmed our results. At the end of the study period, more than a third of our living donors had a moderate to high risk of CKD progression, similar to reports by other authors [31-33]. However, Kishnan et al. [34] demonstrated no higher risk of development of CKD stage 4/5 (eGFR <30 mL/min/1.73 m3) in <10-year follow-up after comparison of live donor datasets from the UK Transplant Registry with the cohort of healthy nondonors from the Health Improvement Network database. The authors emphasized that the British Transplantation guidelines recommend a lifelong follow-up of live donors. Therefore, it is possible that an early diagnosis and intervention of CKD minimizes the risk of end-stage renal disease [34].
Urinary Proteins and Enzymuria
Patients with a solitary kidney should be monitored regularly throughout their lives. Typically, blood pressure, renal function (eGFR), and proteinuria are measured. An increase in arterial hypertension and proteinuria in the long-term course after nephrectomy has been described [35]. The risk of a donor becoming subject to dialysis is controversial [3, 5]. However, most studies evaluating renal function after living donation were limited to the determination of the eGFR, U-Prot, and sometimes urinary Alb levels. The results of different studies are very heterogeneous, which is partly due to differences in the size and composition of the study groups as well as the length of follow-up [2, 3]. Both urinary U-Prot and Alb are significantly increased only during the long-term course (>10 years) after DN [36]. Our study is the first to analyze the course of glomerular and tubular urinary proteins after DN over 1 year.
Consistent with our results, no significant increase in U-Prot or Alb concentrations in the urine, compared to the preoperative values, was detected at 1 year after donation [37]. The observed significant short-term increase in urinary Alb in our study, with a maximum level on the second day after the surgery, was also described by other authors [11, 38]. Hoogendijk-van den Akker et al. [11] hypothesized that the short-term Alb elevation could be caused by glomerular permeability during surgery. No studies have examined the behavior of the high-molecular-weight glomerular urinary proteins IgG and Tf after DN [39]. In our study, similar to Alb, IgG and Tf levels were increased for a short time and then stabilized. The 2-fold increase in the median, with a maximum value on the third day after surgery, was significantly lower than that of Alb (3.5-fold increase). A postrenal cause could be excluded. At the end of the year, the medians of all 3 glomerular urinary proteins were within the reference ranges.
Commonly used markers such as creatinine and U-Prot are not sufficiently sensitive for early detection of kidney damage, particularly injury of the proximal tubule. Markers indicating tubular injury before functional loss, such as low-molecular-weight urinary proteins or urinary enzymes, contribute little to the amount of total protein and are insufficient or not detectable by conventional quantitative or semiquantitative protein analytical methods [12]. If the proximal tubules are dysfunctional, the low-molecular-weight proteins are present in higher levels in the urine [40, 41]. In addition to α1M and β2M, RbP is a sensitive marker of damage in the tubules [42]. Enzymes of tubular origin, such as NAG, a lysosomal high-molecular-mass enzyme, are released into the urine in response to lesions of tubule cells [43]. The amount of enzyme detectable in the urine correlates directly with the tubular disorder [41]. Our findings provide information on tubular kidney damage after DN. This damage is indicated by the extreme increase in tubular markers shortly after the operation (β2M 150-fold, RbP 23-fold, α1M 10-fold, and NAG 3.5-fold) and by the fact that no normalization occurs within 1 year. Argiles et al. [9] reported adaptive changes in the remaining single kidney after DN. The authors explained the significant increase in β2M and RbP excretion by changes in tubule function. Gluhovschi et al. [10] emphasized the importance of tubular lesions of the solitary kidney. In their review, they described the “solitary kidney” under different conditions, among other factors, after living donation. They recommend an extension of monitoring of patients with a solitary kidney, usually consisting of measurements of blood pressure, proteinuria, and eGFR, to the measurement of tubular injury markers. Hoogendijk-van den Akker et al. [11] investigated Alb and α1M after DN. As we also noted, they described the different behaviors of both urinary proteins; Alb was only increased in the short term, while α1M slowly increased to a maximum level on the third day after surgery and remained elevated after 4–6 weeks. In a long-term follow-up study, Meier et al. [44] revealed a significant 6 times higher concentration of α1M 10 years after DN. These results confirmed our findings and indicate tubular dysfunction in the solitary kidney. The tubular markers we examined at 1 year after the surgery were significantly higher than the initial values and above the reference ranges. Similar observations are available for NAG. Elevated NAG values, compared to those of a healthy control group, have been described after DN [43].
Related to the results of Regeniter et al. [13], we also found abnormal urinary protein patterns in patients at low risk to CKD progression. The authors explained that kidney damage may already be present despite normal laboratory findings and before the eGFR is significantly reduced. To avoid overlooking a risk for disease, they recommended a proteinuria analysis that includes both glomerular and tubular kidney markers, especially for high-risk patients [13]. Undoubtedly, this group includes individuals with a single kidney. The determination of the urinary marker proteins Alb (glomerular) and α1M (tubular) during follow-up supports the detection of possible kidney damage early. These results could help to prepare donors to optimize the postdonation outcomes like lifestyle modification for protection of the solitary kidney.
Conclusion
We investigated the influence of DN on the function and status of the remaining solitary kidney up to 1 year after surgery. At the end of the study period, more than a third of our living donors had a moderate to high risk at progression of CKD. The biochemical glomerular and tubular kidney markers examined showed different behaviors. After a transient increase, the glomerular proteins normalized. Conversely, the detection of low-molecular-weight urinary proteins and enzymuria reflected mild tubular damage at 1 year after the DN. Our findings suggest that for the evaluation of mild tubular damage, low-molecular-weight marker proteins should be included in the urine diagnostic of a personalized living kidney donor follow-up. However, further studies are needed to confirm if urinary markers can improve the risk stratification of living kidney donors.
Statement of Ethics
Our project was approved by the Local Ethics Committee of the Martin Luther University Medical School Halle and written informed consent of the living donors obtained (Reference No. 2016-163).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding to declare.
Author Contributions
The individual contribution of each coauthor is as follows: As the corresponding author, Gerit Theil took part in collecting and analyzing the data, drafting the article, and finally approving the study. Kersten Fischer, PhD, and Joanna Bialek, PhD, contributed in analysis and interpretation of data. Karl Weigand, MD, was involved in drafting the manuscript and final approval of the version to be published. Prof. MD Paolo Fornara contributed by drafting and approving the article and conception and design of the study and providing intellectual content of critical importance to the work described. All authors fulfill the criteria of ICMJE authorship, deserve credit, and can take responsibility for the work.