Abstract
Introduction: Aminoglycosides, valued for their lower antimicrobial resistance, are used for perioperative antibiotic prophylaxis (PAP) in urological procedures such as robot-assisted radical prostatectomy (RARP). However, data regarding the safety of gentamicin in robot-assisted surgery remain limited. This study assessed the incidence of acute kidney injury (AKI) associated with PAP with single-dose gentamicin during the transition from open prostatectomy to RARP. Methods: This single-center, retrospective, matched case-control study included 77 RARP patients receiving gentamicin and 72 matched controls receiving cefuroxime. AKI was assessed using the Kidney Disease: Improving Global Outcome (KDIGO) criteria, considering age, comorbidities, and prostate weight. Results: AKI occurred in 33.8% of the gentamicin group versus 9.7% of the cefuroxime group, resulting in an odd’s ratio (OR) of 6.25. In the gentamicin group, grade 1 AKI was most frequent (19.5%), followed by grade 2 (7.8%) and grade 3 (6.5%). In the cefuroxime group, grades 1 (5.6%) and 2 (4.2%) were observed. Prostate volume and gentamicin use emerged as independent cofactors. Limitations include missing long-term data, variable gland measurements, and inclusion of patients with pre-existing kidney disease. Conclusion: The risk of AKI was significantly higher after PAP with gentamicin compared to PAP with cefuroxime (OR: 6.25, 95% CI: 2.095–18.664, p = 0.001), suggesting that PAP with gentamicin should be avoided in RARP.
Plain Language Summary
This study investigated how often patients developed acute kidney problems after robot-assisted prostate removal, comparing the effects of two antibiotics, gentamicin and cefuroxime, given during surgery to prevent infections. It concluded that gentamicin poses a higher risk of kidney damage compared to cefuroxime and therefore should not be used in everyday clinical practice in this surgical context.
Introduction
Antimicrobial resistance is an escalating global challenge, prompting the World Health Organization (WHO) to classify it as one of the top ten public health threats facing humanity [1]. Urological infections rank among the most common conditions requiring antibiotic therapy, yet antimicrobial resistance frequently limits effective treatment options. Commonly prescribed antibiotics are increasingly ineffective against prevalent uropathogens due to high resistance rates [2]. This problem also affects perioperative antibiotic prophylaxis (PAP), where standard regimens are increasingly undermined by bacterial resistance, limiting safe and effective options. The use of broad-spectrum agents is strongly discouraged due to their role in driving further resistance. In contrast, aminoglycosides remain a class of antibiotics with relatively low resistance rates among typical uropathogens, making them a valuable option in this setting [3].
Gentamicin and other aminoglycosides are well known for their antimicrobial potency but are associated with dose-dependent toxicities, particularly nephrotoxicity and ototoxicity, which may lead to acute kidney injury (AKI) or hearing loss when administered over extended periods. As a result, aminoglycosides are prescribed less frequently than other antibiotic classes, which, compared to third-generation cephalosporins, may explain the lower prevalence of resistance in Escherichia coli and other common uropathogens [3]. The administration of a single-dose of gentamicin as PAP is considered a safe and effective option for various urological procedures in the current best practice statement of the American Urological Association (AUA) [4].
At our institution, PAP with a single weight-adjusted dose of gentamicin has been routinely used in open surgeries since 2010. Nevertheless, with the transition from open abdominal to intra-abdominal robot-assisted urological procedures, we began observing cases of AKI following this prophylactic regimen. This study was therefore initiated to investigate the risk of AKI after PAP with single-dose gentamicin in the context of robot-assisted urological surgery.
Methods
We conducted a single-center, retrospective, matched case-control study with 149 patients who underwent robot-assisted radical prostatectomy (RARP) between January 1, 2020, and December 31, 2022. All included patients received PAP 30–60 min before surgery, consisting of either a single weight-adjusted dose of gentamicin (5 mg/kg body weight) or a single standard dose of 1.5 g cefuroxime infused over a period of 30 min in accordance with the recommendations of the American Urological Association [5]. The gentamicin dose was not adjusted in patients with mild-to-moderate renal impairment as nephrotoxicity is associated with cumulative exposure and prolonged therapy rather than with isolated prophylactic dosing, provided there are no severe renal failure (estimated glomerular filtration rate [eGFR] <15–20 mL/min) and no dependency on dialysis [6]. Body weight was measured at the time of admission. All procedures were performed by two experienced surgeons working together, each using their own console. Both were certified according to the criteria of the German Cancer Society.
In all patients, the intra-abdominal carbon dioxide pressure was maintained at 10–12 mm HG and only temporarily increased to 20 mm HG during transection of the Santorini plexus. In the absence of major bleeding, intraoperative blood transfusion was required in approximately 1% of cases in both groups. Lymphadenectomy was performed in all patients. No restrictive fluid management strategy was implemented, and no intraoperative diuretics were administered during surgery. Anesthesia protocols were consistent across both groups.
For the case-control study, patients were stratified into two groups of approximately equal size. Within the context of the antibiotic policy implemented in our hospital, the decisive criterion for patient inclusion was the designated cutoff date, specifically set on July 1, 2021, coinciding with the change of antibiotic regimen. We managed to include every case within a specified timeframe of one and a half years preceding and following the cutoff, ensuring a nearly balanced distribution between the groups.
We assessed the incidence of AKI within 7 days after RARP. According to the KDIGO (Kidney Disease: Improving Global Outcome) criteria [7], AKI was defined as a rise in serum creatinine level by at least 0.3 mg/dL (26.5 μmol/L) within 48 h after surgery or as a 1.5-fold rise above baseline within 7 days after surgery. When multiple serum creatinine values were available, the highest value was used. The severity of AKI was classified from grade 0 (no AKI) to grade 3 according to KDIGO criteria.
Additional potential risk factors such as age and diabetes mellitus were recorded. Postoperatively, the weight of the resected prostate was determined by the pathology department. Another aim was to assess the potential influence of comorbidities on the incidence of AKI. For this purpose, the Charlson Comorbidity Index (CCI) [8] was used as a composite measure. Also, the presence of diabetes mellitus type II (DM2) was evaluated separately.
Statistical analyses were performed using IBM SPSS Statistics (Version 28.0.1.0; 142). Fisher’s exact test, Mann-Whitney U test, and Kruskal-Wallis test for independent samples were used with a significance threshold of p < 0.05. A multivariable logistic regression analysis was conducted to evaluate associations between variables. Adjusted odd’s ratios (OR), confidence intervals (CIs), and p values (threshold p < 0.05) were calculated.
Results
A total of 149 patients were included in the study. Patients received PAP either with gentamicin (n = 77) or cefuroxime (n = 72). In both groups, potential risk factors for AKI such as age (p = 0.381), CCI (p = 0.540), DM2 (p = 0.064), and prostate weight (p = 0.984) were not significantly different (Table 1). Similarly, also preoperative serum creatinine (p = 0.126) and eGFR (p = 0.130) did not differ significantly between both groups, indicating equivalent baseline renal function (Table 1).
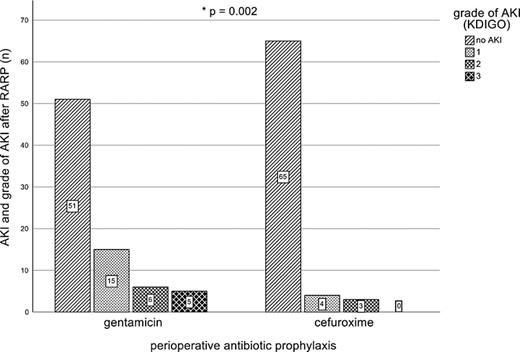
In the gentamicin group, the median age was 67 years (range 45–77 years), the median CCI was 0 (range 0–4), 15.6% of patients had a diagnosed DM2, and the median prostate weight was 50.5 g (range 25.1–238 g). In the cefuroxime group, the median age was 65 years (range 52–77 years), the median CCI 0 (range 0–5), 5.5% of patients had a diagnosis of DM2, and the median prostate weight was 50.5 g (range 27–180 g). Among patients receiving PAP with gentamicin 26 of 77 (33.8%) developed AKI, while 51 patients (66.2%) did not develop AKI. In contrast, in the cefuroxime group, only seven (9.7%) patients out of 72 developed AKI (p = 0.002; Fig. 1).
The majority of AKI cases in the gentamicin group developed grade 1 AKI (n = 15, 19.4%). Grade 2 and grade 3 AKI occurred in six (7.8%) and five (6.4%) patients. In the cefuroxime group, no grade 3 AKI was observed, but 4 patients developed grade 1 AKI, and 3 patients developed grade 2 AKI (Fig. 1).
In the univariate analysis, PAP with gentamicin (p < 0.001), age (p = 0.05), DM2 (p = 0.009), and prostate weight (p = 0.002) were significantly associated with the occurrence of AKI after RARP. Among patients who developed AKI after RARP, 78.8% (n = 26) received a PAP with gentamicin compared to only 44% (n = 51) of patients who did not develop AKI. The median age of patients who developed AKI after RARP was 69 years (range 53–77 years), the median CCI was 0 (range 0–4), 24.2% (n = 8) had a DM2 diagnosis, and the median prostate weight was 60 g (range 32.6–238) compared to a median age of 65 years (range 45–77 years), a median CCI of 0 (range 0–5), a DM2 diagnosis in 24.2% (n = 8), and a median prostate weight of 49 g (range 25.1–180 g), among patients without AKI.
However, multivariate analysis multivariable logistic regression analysis identified only PAP with gentamicin (p = 0.001) and prostate weight (p = 0.008) as significant independent risk factors of AKI. Perioperative prophylaxis with gentamicin was associated with an approximately sixfold (OR: 6.253, 95% CI: 2.095–18.664) increased risk of AKI. An increase of the prostate weight of 1 g was associated with a 2.4% enhanced risk of AKI after RARP (OR: 1.024, 95% CI: 1.006–1.041). In contrast age, CCI and DM2 did not emerge as significant independent predictors of AKI in the multivariable model. The OR for the risk factor age was 1.045 (95% CI 0.972–1.124, p = 0.230), for CCI 1.176 (95% CI 0.701–1.972, p = 0.538), and for DM2, 2.388 (95% CI 0.590–9.666, p = 0.222).
Discussion
The current study identified PAP with gentamicin and prostate weight (OR: 1.024, 95% CI: 1.006–1.041) as main risk factors for the development of AKI after RARP. AKI occurred over six times more frequent after PAP with gentamicin compared to cefuroxime (OR: 6.253, 95% CI: 2.095–18.664). Despite most cases of AKI after PAP with gentamicin appeared to be grade 1 (n = 15, 19.4% of patients), also grade 2 and grade 3 AKI were observed in six (7.8%) and five (6.4%) patients, respectively. In the control group which received PAP with cefuroxime, 4 patients developed grade 1 AKI, and 3 patients grade 2 AKI. Grade 3 AKI was absent in the control group.
Preoperative serum creatinine and eGFR values did not differ significantly between the groups, indicating comparable baseline renal function. This equivalence is a key prerequisite for the internal validity of the study, as it minimizes potential confounding due to pre-existing renal impairment. It is therefore likely that any differences observed in postoperative renal function parameters can be primarily attributed to the perioperative intervention – particularly the choice of antibiotic prophylaxis.
Hayward et al. [9] reviewed the adverse effects of a single dose of gentamicin in adults. Approximately 10% of the included 24,107 participants receiving single dose of gentamicin, mostly as perioperative surgical prophylaxis, later developed AKI. Another meta-analysis by Srisung et al., which included 18,354 patients, only identified a gentamicin-containing surgical prophylaxis as a significant risk for developing postoperative AKI in orthopedic surgery with a relative risk of 2.99 (95% CI: 1.84, 4.88) [10]. In our study of perioperative prophylaxis in RARP, individuals who received gentamicin had a significant more than sixfold higher risk of developing AKI compared to those who did not receive PAP with gentamicin. The strong association between gentamicin usage and AKI emphasizes the potential risk associated with the use of this antibiotic.
Sato et al. [11] assessed the incidence of AKI in patients who underwent RARP and reported early AKI (immediately postoperatively) and late AKI (days one to seven postoperatively) in 46.9% and 3.9% of patients, respectively. Similar findings were reported by Naito et al. [12] who observed transient AKI after RARP in 13.8% of patients. These results suggest that AKI rates are markedly higher, if the renal function is tested immediately after surgery, but lower if assessed later between days one and seven after surgery. Joo et al. [13] investigated the onset of AKI after RARP and radical retropubic prostatectomy and found a rate of 5.5% of (probably late) AKI after RARP. In our study, we specifically evaluated the presence of late AKI in the sense of the above definition at the day after surgery and found that 33.8% of patients in the gentamicin group showed some degree of AKI according to KDIGO criteria. Thus, in our study, AKI after PAP with gentamicin occurred much more frequent compared to the previous studies discussed above. Unfortunately, none of the referenced studies provided details about the PAP used, thus limiting direct comparability with our study. It is plausible that the increased intra-abdominal pressure during RARP impairs renal perfusion, thereby promoting accumulation of gentamicin within the renal parenchyma and enhancing its nephrotoxic potential. Taken together, the occurrence of AKI in the setting of RARP appears to require the presence of additional renal stressors. In this context, even a single prophylactic dose of gentamicin appears to exert a particularly pronounced nephrotoxic effect.
In the multivariable logistic regression analysis, the only other independent significant risk factor for the development of AKI was prostate weight. An increase of the prostate weight of 1 g correlated with a 2.4% increased risk to develop AKI after RARP (OR: 1.024, 95% CI: 1.006–1.041). This suggests that the size of the prostate gland is a risk factor for the occurrence of postoperative AKI. Although the underlying mechanism remains unclear it is noteworthy that surgery for larger prostate glands was slightly, but not significantly longer in both groups. While the exact duration of the pneumoperitoneum at 20 mm Hg was not documented, it is conceivable that larger prostates required more extensive dissection and hemostasis of the periprostatic venous plexus. A prolonged high-pressure phase of the procedure could lead to transient renal hypoperfusion and amplification of the nephrotoxic effect of gentamicin. Although the underlying mechanism remains speculative, this hypothesis offers a plausible explanation for the observed association and underscores the need for further research on intraoperative factors affecting renal outcomes. Modi et al. [14] compared the eGFR between an experimental and a control group (88.4 vs 85.0 mL/min/1.73 m2, p = 0.11) during RARP and concluded that an increased pressure in the pneumoperitoneum of 20 mm HG does not significant increase the risk of renal complication. Ferroni et al. [15] found that lower intra-abdominal pressure of 6 mm HG compared to standard pressure of 15 mm HG is manageable in RARP without increasing the risk of complications (4.0% vs. 8.7%, p = 0.02). Based on our hypothesis of intrarenal gentamicin accumulation and increased toxicity, it is conceivable that a reduction of the intra-abdominal pressure during RARP might reduce the incidence of postoperative AKI. To date, no specific studies have addressed that issue and, given the results of our study, a prospective trial to directly test this hypothesis would likely not be ethically justifiable.
In view of the known frequent nephrotoxic side effects of gentamicin, it is important to discuss the requirement of antimicrobial prophylaxis in RARP. The European Association of Urology (EAU) guidelines do not recommend the minimal invasive procedure over other approaches, citing no significant differences in terms of oncological outcomes [16, 17]. However, as robot-assisted techniques are increasingly chosen at the patient request, RARP is progressively replacing the open approach in clinical practice. Consequently, antibiotic prophylaxis protocols originally designed for open surgery may no longer be fully appropriate for the robotic setting. A large-scaled systematic review by Moretti et al. [18] confirmed that minimal invasive surgery and particularly RARP offers superior perioperative as well as complication-related outcomes. In this evolving context, it is important to evaluate potential risks associated with antibiotic choices. To date, no specific prophylactic antibiotic has been universally mandated for RARP, largely due to a lack of high-level evidence. Clinicians typically follow local guidelines, which take into account regional resistance patterns and antibiotic availability. According to current recommendations, single-dose gentamicin is listed as a second-line agent for PAP in patients with beta-lactam intolerance [4]. However, gentamicin-related nephrotoxicity in the context of RARP is likely underreported, as its use is generally limited to isolated cases with beta-lactam intolerance. Nephrotoxic events are rare under these circumstances. In our study, no postoperative infections occurred in either group, indicating that both gentamicin and cefuroxime provided equivalent antimicrobial efficacy with respect to the relevant spectrum of pathogens. Nonetheless, our findings suggest that current PAP recommendations warrant reevaluation, particularly regarding the use of gentamicin as an alternative in cases of standard prophylaxis intolerance. As a consequence of this study, our institution replaced gentamicin with clindamycin as second-line agent for PAP in patients undergoing RARP.
This study has several limitations. A general limitation is that the effect of single-dose PAP with gentamicin on postoperative renal function was assessed only in the context of RARP and not compared to patients undergoing conventional open radical prostatectomy. Therefore, we cannot provide a direct comparison of the renal toxicity of single preoperative dose of gentamicin in both surgical settings. Another limitation is the absence of long-term follow-up data on renal function. Due to the relatively short follow-up period, we have no data on the need of dialysis or the time required until full recovery of kidney function. In our setting, follow-up laboratory testing is typically managed by general practitioners, while the hospital-based prostate center primarily focuses on continence management, oncological surveillance, and the assessment of erectile function. However, it can be assumed that a renal recovery phase has commenced approximately 4 weeks following the treatment, indicating at least partial regeneration of kidney injury. [19]. Preoperative prostate volume was inconsistently documented via ultrasound, and in some cases, gland weight was missing from pathology reports. Patients with chronic kidney disease (CKD) were not excluded. Distribution of patients with CKD was, however, equally distributed with each group including 10 patients with CKD (6.7%). Only 3 patients (2%) showed further deterioration in renal function by the time of discharge. Despite the presence of AKI cases in the gentamicin group, we did not exclude CKD patients, as the small number of cases lacked sufficient statistical power to support causal inferences.
Nonetheless, CKD may represent a predisposing factor for AKI when a gentamicin-based PAP is used in RARP. In conclusion, AKI occurred significantly more frequently following RARP when PAP with gentamicin was used compared to PAP with cefuroxime. Body weight-adapted single-dose gentamicin should therefore not be used in the context of robot-assisted surgery, neither as first-line choice nor as an alternative. These findings support the need for a reevaluation of current guideline recommendations regarding antibiotic prophylaxis in minimally invasive urological procedures.
Statement of Ethics
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by Ethik-Kommission der Otto-von-Guericke-Universität an der Medizinischen Fakultät und am Universitätsklinikum Magdeburg A.ö.R. – approval: 04.11.2022. All adult participants provided written informed consent to participate in this study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
M.S. and S.B. are the center surgeons who jointly performed all the operations in this study. M.G., M.S., S.B., and G.G. were involved in data analysis, study design, and preparation of the manuscript. A.L. performed the statistical analysis and was involved in the preparation of the manuscript. All authors critically revised the final version.
Additional Information
Michael Glietsch and Simon Blaschke contributed equally as first authors, and Gernot Geginat and Martin Schostak contributed equally to this work.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.