Abstract
Introduction: Fumarate hydratase-deficient renal cell carcinoma is a rare and aggressive subtype associated with hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, characterized by germline mutations in the fumarate hydratase (FH) gene. Here, we report a case of HLRCC with early recurrence during adjuvant therapy following radical nephrectomy. Case Presentation: A 34-year-old woman with FH-deficient RCC presented with fever, right flank pain, and a large renal mass with a tumor thrombus. Open radical nephrectomy and IVC tumor thrombectomy were performed. Pathological findings and genetic analyses confirmed the diagnosis of HLRCC. Despite adjuvant pembrolizumab therapy after nephrectomy, bone metastases were detected within 9 weeks. The patient was treated with stereotactic body radiotherapy (SBRT), followed by systemic therapy with nivolumab and cabozantinib. After 16 months since the recurrence, no further disease progression was observed. Genetic counseling revealed the same FH mutation in her daughter, prompting annual surveillance. Conclusion: This case highlights the potential efficacy of combining tyrosine kinase inhibitor therapy with SBRT in managing aggressively progressive HLRCC.
Introduction
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) is an autosomal dominant disorder characterized by major clinical manifestations, including cutaneous leiomyomas, uterine leiomyomas, and RCC. HLRCC is caused by pathogenic germline variants in the fumarate hydratase (FH) gene [1, 2]. HLRCC-associated RCC is highly aggressive, prone to early metastasis, and can occur even in young individuals [3]. Because of its rarity, no definitive treatment strategy has been established. We report a case of HLRCC-associated RCC with bone metastases shortly after adjuvant pembrolizumab immunotherapy postoperatively in which no disease progression was observed after transitioning to combination therapy with nivolumab (NIVO) and cabozantinib, along with stereotactic radiation therapy. Furthermore, through genetic testing, we highlight the importance of managing hereditary tumors in young patients and offer insights into treatment strategies.
Case Presentation
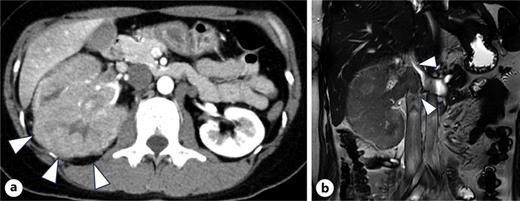
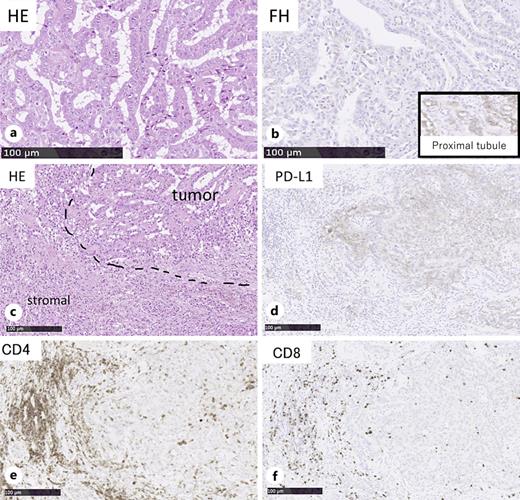
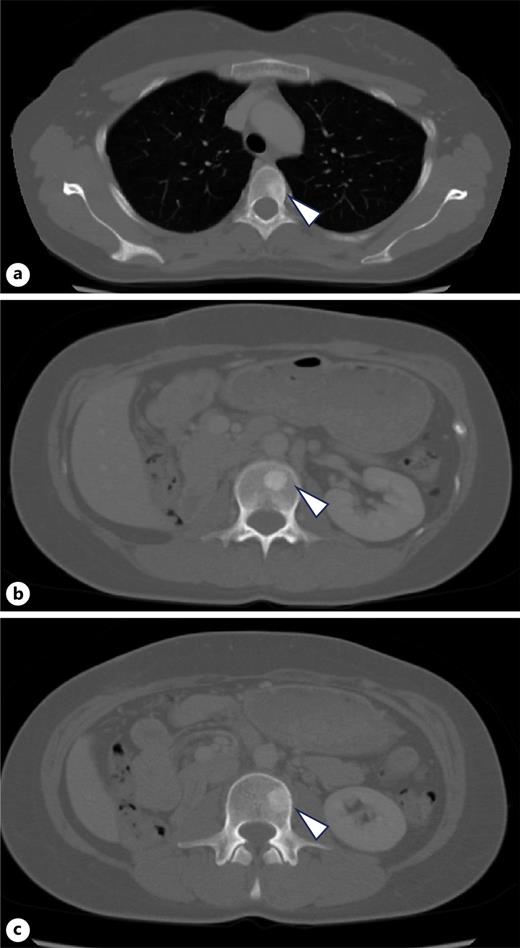
A 34-year-old woman presented with fever and right flank pain. Contrast-enhanced computed tomography (CT) revealed a 116 × 83 mm poorly enhanced solid mass replacing the right kidney, suspicious for RCC and tumor thrombus in the inferior vena cava below the hepatic vein inflow without apparent lymph node or distant metastases (shown in Fig. 1). Brain magnetic resonance imaging (MRI) showed no evidence of brain metastases, and bone scintigraphy revealed no abnormal uptake. The patient’s medical history included depression, and family history was notable for epilepsy in her brother and daughter. Therefore, the patient was diagnosed with non-clear cell RCC at the clinical stage cT3bN0M0. Concurrently, uterine leiomyomas were observed, along with a family history, raising suspicion for HLRCC. Open radical nephrectomy and inferior vena cava tumor thrombectomy were performed. Pathological findings revealed atypical tumor cells with irregular glandular structures and large nucleoli resembling nuclear inclusions. Immunohistochemical analysis showed loss of FH protein in the tumor cells, confirming the diagnosis of FH-deficient RCC, pT3bN0M0, stage III, WHO/ISUP grade 3 (shown in Fig. 2). PD-L1 expression and CD4/CD8-positive lymphocyte infiltration were observed, although most infiltrating lymphocytes were stromal with minimal intratumoral infiltration. Four weeks postoperatively, CT showed no recurrence. Adjuvant immunotherapy with pembrolizumab was initiated owing to the pT3b stage. Genetic counseling was performed with informed consent. Gene sequencing using a hybrid capture method identified a missense variant of FH c.698G>A (p.Arg233His) [NM_000143.4]. According to the ClinVar database (variation ID: 16236), the clinical significance of FH c.698G>A is pathogenic. Combined with her phenotype, we confirm the diagnosis of HLRCC. At week 9 of pembrolizumab therapy, CT revealed bone metastases at Th4, L2, and L3 (shown in Fig. 3). After discussing the available treatment options along with their respective benefits and drawbacks, the patient chose to pursue the most intensive therapy despite potential side effects. These oligometastases were treated with stereotactic body radiotherapy (SBRT) (24 Gy in two fractions). Therefore, systemic therapy with NIVO (240 mg) and cabozantinib (40 mg) was initiated. The patient remained stable disease for 16 months since the recurrence. Genomic testing using the FoundationOne CDx® panel identified the known FH mutation and genetic abnormalities in PIK3R1 and TP53; however, no additional actionable mutations were detected. Mutations in BRCA1/2 have been identified; however, these have been classified as variants of uncertain significance. After undergoing multidisciplinary genetic counseling, the patient consented to genetic testing of her 9-year-old daughter, which revealed the same FH gene mutation. Consequently, annual MRI screening of pediatric patients was initiated.
Discussion
Here, we report a case of FH-deficient RCC associated with HLRCC that developed early bone metastases during adjuvant pembrolizumab therapy following radical nephrectomy. Knowingly, there are no previously reported cases of recurrence during adjuvant therapy for FH-deficient RCC. Although HLRCC typically has poor prognosis with the potential for rapid disease progression, the patient responded well to subsequent multimodal therapy. Here, we report the clinical course, pathological findings, and treatment options of this case.
FH-deficient RCC is a rare subtype of RCC defined by the loss of FH function due to either germline or somatic mutations. HLRCC is definitively diagnosed if a pathogenic FH germline variant is located on chromosome 1q42-44. The median age of onset for FH-deficient RCC associated with HLRCC is 40 years, with men more frequently affected than women (male/female ratio; 2.3:1). It is highly aggressive with poor survival in stage 3–4 disease, with a median survival period of 18 months [4]. FH-deficient RCC can be detected based on negative IHC results using anti-FH antibodies. IHC with an anti-FH antibody was associated with a sensitivity of 86.0–87.5% and a specificity of 100% [5, 6].
The mechanism of FH-deficient RCC has traditionally been attributed to the accumulation of fumarate inducing HIF-1α and subsequent pathway [7]. Recent studies have implicated mitochondrial DNA leakage in inflammation, further complicating the disease pathology [8].
Systemic treatment options for metastatic FH-deficient RCC are limited. Dual inhibition of tyrosine kinase inhibitors (TKIs) demonstrated superiority over mTOR inhibitors or ICIs alone, achieving an ORR of approximately 64% and a time to progression of 11.6 months [9]. Cabozantinib is the preferred TKI for HLRCC [10]. Notably, a case of 31-month complete response with combined ipilimumab (IPI) and NIVO therapy has been reported [11]. In a relatively large, multicenter retrospective study involving 77 patients, combined ICI and TKI therapy demonstrated more favorable overall survival (HR; 0.19, 95% CI; 0.04–0.90) and progression-free survival in first-line therapy (HR; 0.22, 95% CI; 0.07–0.71) compared to combined bevacizumab and erlotinib therapy [12]. Although the review did not establish the superiority of specific regimens, it highlighted the consistent efficacy of cabozantinib in managing bone metastases [13]. In our case, bone metastases developed during pembrolizumab treatment, and we opted for a combination therapy with NIVO and cabozantinib instead of IPI and NIVO. The effectiveness in this case is likely attributable to systemic therapy and the added benefit of SBRT. SBRT has demonstrated high local control rates of 89–92% for bone metastases in RCC, with minimal toxicity reported across retrospective and prospective studies [14‒16].
Immunohistochemical analysis revealed that the tumor cells were positive for PD-L1 expression, but no significant TILs were present, classifying the tumor as type III, indicative of immune ignorance [17]. The pathological findings of this case suggest that the therapeutic effect of ICI is limited. The poor prognosis and young age of the patient supported the proposal of adjuvant ICI therapy, which was initiated with the patient’s consent. Alaghehbandan et al. [18] reported no consistent association between PD-L1 expression and TILs in FH-deficient RCC. Furuya et al. [19] reported that 10 of 13 HLRCC-associated RCCs were positive for PD-L1 expression. Sekito et al. [11] reported that despite negative PD-L1 expression and TILs, combined IPI and NIVO therapy was effective. Evaluation of the immune microenvironment was useful in this case; however, it emphasized the possibility that it may not reliably predict treatment outcomes, highlighting the need for advanced models that integrate pathological and biomarker analyses.
In this case, comprehensive genomic profiling revealed no additional mutations. As of September 2024, 18 cases, including the present case, have been registered in the Cancer Genome Information Management Center database of Japan. Mutations were detected in the FH, NF2, SPEN, KEL, ERBB2, and NOTCH1 genes. However, none of these patients harbored driver mutations that could serve as potential therapeutic targets. In a previous report of 57 tumor samples, NF2, CDH1, and PIK3CA mutations were the most frequent (11%), and 18% of the cases showed deletions in DNA damage repair pathway genes [12]. Further accumulation of genetic mutation data is needed to advance our understanding of this disease and foster the development of novel therapies.
This case highlights the importance of genetic counseling and need for a multidisciplinary approach for comprehensive psychological care. Genetic testing revealed that the patient’s daughter harbored the same FH mutations. Consequently, a surveillance protocol, including annual MRI, was initiated under the guidance of pediatric specialists. Such proactive involvement in multiple disciplines is essential to mitigate the risks to family members who may be affected. Systemic therapy will be continued under the guidance of a multidisciplinary team as long as the patient wishes to pursue intensive treatment. Psychiatric care and logistical support for her daughter’s hospital visits will also be provided.
The immunological evaluation of the tumor and patient’s treatment course suggest the potential efficacy of combined TKI therapy and SBRT. However, further accumulation of similar cases is required to validate these findings and establish more definitive treatment strategies.
Acknowledgments
This case report was supported by all relevant medical staff at Tohoku University Hospital, Seiryo-Machi, 1-1 Aoba-Ku, Sendai, Japan. We are also grateful to the Personalized Medicine Center at Tohoku University Hospital for their valuable support with the genetic analysis.
Statement of Ethics
In accordance with the journal’s guidelines, we have included the corresponding CARE Checklist as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000547393). This study was reviewed and approved by the Ethics Committee of Tohoku University Hospital (Approval No. 35403). The use of the Cancer Genome Information Management Center database of Japan is 2024-1-161. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
This study was not supported by any sponsors or funders.
Author Contributions
Hiroshi Masuda wrote the original manuscript draft. Tomonori Sato: conceptualization, writing, review, and editing. Satoko Sato: investigation. Takuro Goto, Hiromichi Katayama, Yohei Satake, Takuma Sato, Yoshihide Kawasaki, Naoki Kawamorita, and Hidekazu Shirota: data curation. Akihiro Ito: supervision.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy restrictions but are available from the corresponding author upon reasonable request.