Abstract
Introduction: The aim of this study was to present the surgical technique and clinical outcomes of modified ileal conduit for pelvic lipomatosis (PL). Methods: From 2020 to 2022, we prospectively enrolled 9 patients with PL undergoing modified ileal conduit. The patient characteristics, perioperative variables, and follow-up outcomes as well as the description of surgical technique were reported. Results: All 9 patients successfully completed the operation. Two patients had perioperative complications of Clavien-Dindo grade I. The mean operation time and bleeding volumes were 253 ± 51.4 min and 238.9 ± 196.9 mL, with a mean postoperative follow-up time of 13.0 ± 5.6 months. The postoperative 3-month and 1-year creatinine values were significantly decreased versus the preoperative (p = 0.006 and p = 0.024). The postoperative 3-month and 1-year estimated glomerular filtration rate values were significantly increased compared with those before operation (p = 0.0002 and p = 0.018). The separation value of left renal pelvis collection system after operation was significantly reduced compared with preoperative evaluation (p = 0.023 at 3 months and p = 0.042 at 1 year) and so was the right side (p = 0.019 and p = 0.023). Conclusion: Modified ileal conduit is safe and feasible for PL. A large sample cohort with long-term follow-up is needed to evaluate the clinical outcomes of PL.
Introduction
Pelvic lipomatosis (PL) is a rare disease with unclear etiology. It is characterized by excessive growth of pelvic adipose tissue that can compress the bladder, ureter, rectum, and blood vessels, leading to a series of complications such as dysuria, glandular cystitis, hydronephrosis, renal failure, and constipation. The reported incidence rate of PL is 0.6 to 1.7/10 million [1]. Due to the atypical symptoms of PL, patients with PL may be misdiagnosed in the early stage, and the incidence in real world could be higher than the estimation. Urinary diversion is recommended when PL patient experiences persistent hydronephrosis after ureteral stent placement, significant changes in bladder morphology, severe damage to the lower urinary tract function, or unsuccessful ureteral stent placement [2]. Urinary diversion includes percutaneous nephrostomy, ureterocutaneostomy, ureteral reimplantation, ileal conduit [1, 3‒7], but the ideal surgical treatment still remains unclear.
Patients receiving traditional ileal conduit or ureteral reimplantation may experience recurrent ureteral stricture caused by continued growth of pelvic fat since the position of ureteral-ileal or ureter-bladder anastomosis is close to the pelvic cavity [4, 8‒10]. In additional, traditional ileal conduit for PL remains technically challenging with undetermined outcomes. Herein, we describe the experience and novel technique of modified ileal conduit for PL with complete follow-up and outcome assessment.
Methods
Study Design
This is a prospective, multicenter, observational study. This study had been approved by the Institutional Review Board of Peking University First Hospital (approval number: 2019-SR-134). All patients provided informed consent to participate in the study.
Setting and Participants
We prospectively enrolled 9 PL patients from Peking University First Hospital, Beijing Jiangong Hospital, and Emergency General Hospital, who underwent modified ileal conduit from 2020 to 2022.
Preoperative Assessment
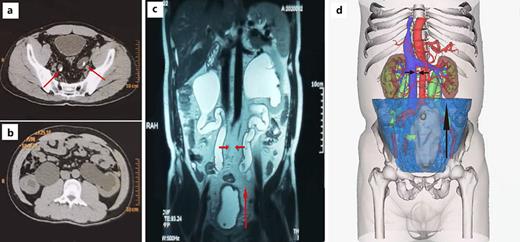
Patients with suspected PL performed blood routine examination, urine routine examination, renal function, liver function, B-ultrasound, CT, MRI, renal dynamic examination, urine flow rate, and urodynamic examination. The diagnosis of PL was made based on B-ultrasound, MRI, CT, or three-dimensional reconstruction models of CTU, showing excessive pelvic fat deposition, deformation of the bladder by extrinsic fat compression, and the ureters which were shifted to the middle [11] (Fig. 1a–d).
Procedure
After all examinations are completed and evaluated, all 9 patients received modified ileal conduit (Fig. 2). Perioperative complications were evaluated by Clavien-Dindo grade. The surgical indications included: severe changes in bladder morphology, severe impairment of lower urinary tract function, persistent hydronephrosis after ureteral stent placement, or failure of ureteral stent placement. During the operation, a ureteral stent was placed on both sides of the ureter and was removed 6–8 weeks after the operation.
Surgical Technique
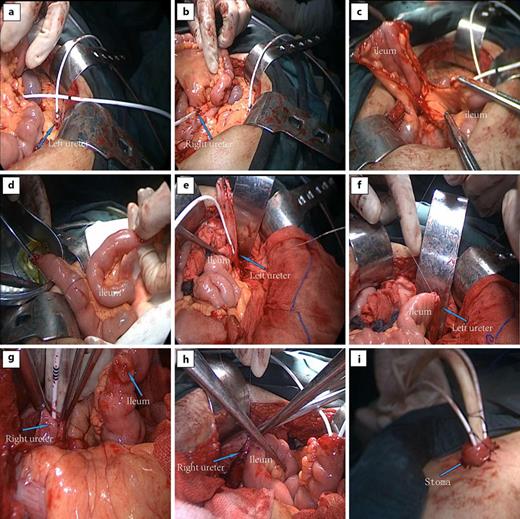
The patient was placed in supine position after general anesthesia and tracheal intubation. After entering the abdominal cavity through a 15 cm midline incision around the navel, the left and right ureters were fully dissociated to the level of the lower pole of the kidney. The ureters were opened, and a ureteral stent was placed, respectively (Fig. 3a, b). A 25 cm-long ileum was isolated and excised near 15 cm from the ileocecal part to make the ileal conduit and the continuity of the original bowel was restored (Fig. 3c, d). At the level of the lower pole of the left kidney, the incised left ureteral orifice was anastomosed end-to-end with the proximal ileal conduit (Fig. 3e, f). A small hole was cut near 10 cm from the proximal end of the ileal conduit (Fig. 3g), and the incised right ureteral orifice was anastomosed with the ileal conduit at the level of the lower pole of the right kidney (Fig. 3h). The distal end of the ileal conduit and the ureteral stent were pulled out of the abdominal wall together, and the distal end was sutured into a papillary shape and fixed with the abdominal wall (Fig. 3i).
Follow-Up
Our patients are on regular follow-up with blood routine examination, urine routine examination, renal function, B-ultrasound initially at 3 months and annually thereafter till the end of the study period.
Statistical Analysis
Quantitative variables were represented as mean ± standard deviation (x ± s). Classified variables were expressed as n (%). GraphPad Prism 9.0.0 software was used for statistical analysis, and paired t test was used to compare the differences of creatinine, estimated glomerular filtration rate (eGFR), and separation value of double renal pelvis collection system before and after the surgery. p value <0.05 was considered statistically significant.
Results
Demographics Data
A total of 9 PL patients underwent modified ileal conduit, including 7 from Peking University First Hospital, 1 from Beijing Jiangong Hospital, and 1 from Emergency General Hospital. All patients are male, with a mean age of 38.4 ± 8.4 years, and mean body mass index of 25.6 ± 3.6 (Table 1). Their first symptoms were various, including dysuria, bilateral low back pain, bladder irritation, hypertension, lower abdominal pain, and so on. All of them had cystitis glandularis (Table 2). CT showed increased fat around the bladder, compressed bilateral ureters, and severe hydronephrosis in bilateral renal pelvis and ureters (Fig. 1a, b). MRI and three-dimensional reconstruction models of CTU indicated that the bladder was deformed and elongated and that the ureter was shifted to the middle (Fig. 1c, d).
Perioperative and Follow-Up Results
Nine patients with PL underwent modified ileal conduit, all of which were performed through an open approach. During the perioperative period, two patients had complications, and their Clavien-Dindo grade was 1. The mean operation time and bleeding volumes were 253 ± 51.4 min and 238.9 ± 196.9 mL, with a mean hospital stay of 13.4 ± 4.8 days (Table 3). The mean postoperative follow-up time was 13 ± 5.6 months. The mean creatinine before surgery, 3 months, and 1 year after surgery were 171.3 ± 57.1 μmol/L, 122.5 ± 21.9 μmol/L, and 123.6 ± 28.0 μmol/L, respectively (Table 3). The postoperative 3-month (p = 0.006) and 1-year (p = 0.024) creatinine levels were significantly decreased than that before operation. The mean eGFR before surgery, 3 months and 1 year after surgery were 59.6 ± 23.4 mL/min×1.73 m2, 77.0 ± 22.1 mL/min×1.73 m2, and 80.4 ± 30.1 mL/min×1.73 m2, respectively (Table 3). The postoperative 3-month and 1-year eGFR values were significantly increased versus those before operation (p = 0.0002 and p = 0.018).
The mean separation value of left and right renal pelvis collection system were 3.7 ± 2.7 cm and 3.6 ± 2.9 cm before operation, which were decreased to 1.8 ± 1.0 cm and 1.3 ± 0.9 cm 3 months after surgery, as the values of 1 year after surgery were 1.2 ± 1.0 cm and 0.8 ± 1.0 cm (Table 3). The separation value of left renal pelvis collection system 3 months (p = 0.023) and 1 year (p = 0.042) after operation were significantly reduced compared with preoperative evaluation and so was the right side (p = 0.019 and p = 0.023).
Discussion
PL is a rare benign disease characterized by excessive deposition of fat in the pelvic cavity leading to compression of pelvic organs, resulting in a series of urinary and gastrointestinal symptoms. In 1959, Engels et al. [12] reported for the first time that a large amount of adipose tissue developed in the pelvic cavity in 5 patients. Afterward, Fogg et al. [13] first introduced the concept of PL.
Although PL is considered to be a benign disease, overgrowth of adipose tissue can lead to the wrapping of the distal ureter. If not treated in time, it can lead to upper urinary tract obstruction and subsequent renal failure [14‒16], which seriously affects the quality of life of patients. At present, the main therapeutic strategies of PL include conservative treatment and surgical treatment [4, 17‒19]. Urinary diversion is recommended when PL patient experiences persistent hydronephrosis after ureteral stent placement, significant changes in bladder morphology, severe damage to the lower urinary tract function, or unsuccessful ureteral stent placement. Urinary diversion includes percutaneous nephrostomy, ureterocutaneostomy, ureteral reimplantation, ileal conduit [1, 3‒7].
In 2019, Ge et al. [4] reported 8 patients with PL treated by pelvic fat clearance combined with ureteral reimplantation. One patient underwent radical cystectomy and ileal conduit due to recurrence of hydronephrosis 49 months after ureteral reimplantation. In 2020, Sanjay et al. [1] represented that 5 patients with PL underwent robot-assisted laparoscopic wide bladder fat extirpation and bilateral ureteral reimplantation, but the study was limited by small sample size and short follow-up time. Flor et al. [3] reported a 43-year-old man with bilateral distal ureteral obstruction secondary to PL. The patient underwent percutaneous nephrostomy to prevent further renal injury.
In 1968, Fogg LB et al. [13] first reported that ileal conduit was used to treat PL patients with severe obstruction of the lower ureters. The operation process was difficult, but during the 2-year follow-up, the patients felt good without any discomfort. Buitrago S et al. [6] represented three PL patients with irritative lower urinary tract symptoms. Three patients received Bricker Wallace type II urinary diversion and well controlled irritative symptoms. Xu T et al. [20] performed ileal conduit on a pair of brother patients with PL complicated with renal failure and upper urinary tract hydronephrosis, and the postoperative creatinine decreased. Yang et al. [10] reported a male patient with PL who underwent traditional Bricker ileal conduit operation. One year after operation, the left ureteroileal anastomotic stricture occurred again due to the further growth of pelvic fat.
Urinary diversion for PL patients remains a technical challenge for urologists. Based on previous literature [4, 8‒10] and our experience, PL patients undergoing traditional ileal conduit or ureteral reimplantation may experience recurrent ureteral stricture caused by continued growth of pelvic fat since the position of ureteral-ileal or ureter-bladder anastomosis is close to the pelvic cavity. To avoid the recurrent ureteral stricture caused by continued growth of pelvic fat, we designed a modified ileal conduit surgery for PL patients who need urinary diversion, which has its own advantages. First, high urinary drainage in the lower poles of both kidneys can avoid recurrent ureteral stricture caused by the continuous growth of pelvic fat. Second, due to the high position of the anastomosis, we do not need to remove the patient’s pelvic fat, avoiding the possible complications, including infection, bleeding, and injury of pelvic organs caused by the removal of pelvic fat. In addition, it has been acknowledged that removal of adipose tissue has limited efficiency in patients with PL [19, 21‒23]. Preserving the PL patient’s pelvic fat can also save the operation time. According to the results of this study, our modified ileal conduit has indeed improved the patient’s renal function and alleviated bilateral hydronephrosis with fewer complications. If the pathology shows that the PL patient has mucinous adenocarcinoma of the bladder and lymph nodes, PET-CT examination can be performed to determine whether the gastrointestinal tract and liver are affected [24]. In addition, adjuvant chemotherapy can be administered to PL patients with bladder tumor and close follow-up is important. During our follow-up period, no cases of recurrent ureteral stricture or aggravation of hydronephrosis were observed, which further proves that our modified ileal conduit is a safe and effective technique for PL patients who need to undergoing urinary diversion.
This study certainly had some limitations. Our study did not compare the therapeutic efficacy and safety of traditional ileal conduit, ureteral replantation, and our modified ileal conduit. The study is also limited by its small sample size and short follow-up time. Despite these limitations, we believe that our experience with modified ileal conduit demonstrates a safe and feasible option for PL.
Conclusions
In this study, a modified ileal conduit surgery was used for the surgical treatment of PL, which could be an efficient and safe surgical technique. A large sample cohort with long-term follow-up is needed to evaluate the clinical outcomes of PL.
Acknowledgment
We would like to thank the Peking University First Hospital, Beijing, China.
Statement of Ethics
This study was reviewed and approved by the Institutional Review Board of Peking University First Hospital (approval number: 2019-SR-134). All patients provided written informed consent to participate in the study and for the publication of their details in the article and all figures and tables in it. All methods were performed in accordance with the relevant guidelines and regulations.
Conflict of Interest Statement
The authors have no relevant financial or non-financial interests to disclose.
Funding Sources
This work was supported by Wuxi “Taihu Talents Program” Medical and Health High-level Talents Project.
Author Contributions
Mancheng Xia, Peng Zhang, and Xuesong Li conducted the literature search, performed the surgery, and wrote the manuscript. Xuesong Li, Kai Zhang, Ninghan Feng, and Liqun Zhou designed the study. Kai Zhang and Chang Meng provided surgical illustrations. Xiaohui Tan, Yuke Chen, Hongjian Zhu, Kunlin Yang, and Jian Fan revised the manuscript. Zhihua Li and Bing Wang collected clinical data. All authors read and approved the final manuscript.
Additional Information
Mancheng Xia, Chang Meng, and Peng Zhang contributed equally to this work.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Further inquiries can be directed to the corresponding author.