Abstract
Succinate dehydrogenase (SDH)-deficient renal cell carcinoma (RCC) is a new subtype of RCC included in the 2016 edition of the WHO classification in RCC. SDH-defective RCC accounts for 0.05–0.2%, and preoperative diagnosis is difficult. We report a severe adherent RCC of inferior vena cava that underwent open radical nephrectomy after preoperative renal artery embolization. Postoperative histopathological examination diagnosed SDH-defective RCC; the clinicopathological stage was pT2b. After 10 months of follow-up, the patient had no evidence of disease recurrence. For patients with large RCC, interventional embolization can be selected to reduce intraoperative bleeding and blood transfusion, and it is recommended to complete interventional surgery within 3–4 h before surgery. SDH-deficient RCC is difficult to distinguish from other renal tumors in imaging, so immunohistochemical examination of SDHB is recommended for young and middle-aged patients, especially those under 45.
Introduction
Succinate dehydrogenase (SDH)-deficient renal cell carcinoma (RCC) is a new subtype of RCC included in the 2016 edition of the WHO classification in RCC. SDH-defective RCC accounts for 0.05–0.2%, and preoperative diagnosis is difficult [1]. We report a very rare case of RCC:SDH-deficient giant RCC in a young man. Due to the rarity of the disease and little previous research and experience, the purpose of this study was to share the clinical features, diagnosis, treatment, and prognosis of giant SDH-deficient RCC.
Case Report
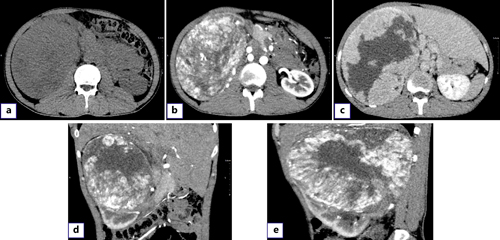
A 28-year-old male was admitted with pain in the right abdomen and gross hematuria. His blood pressure and temperature were normal. Palpation of the abdomen revealed a large mass in the right upper abdomen with a hard texture and poor mobility. The patient had no previous history of gastrointestinal diseases and no family history of tumors. Leukocytes (12.19 × 103/μL) and hypersensitive C-reactive protein (118.73 mg/L) were increased. Urinalysis showed increased RBC (24,158.3/HPF) and white blood cell count (612.2/μL). Enterococcus faecalis was found in urine culture. Contrast-enhanced computed tomography showed a 175 × 130-mm heterogeneous mass in the upper pole of the right kidney, with multiple low-density and nodular calcification shadows. The arterial mass was significantly enhanced and was supplied by the right renal artery without metastasis (Fig. 1a–e).
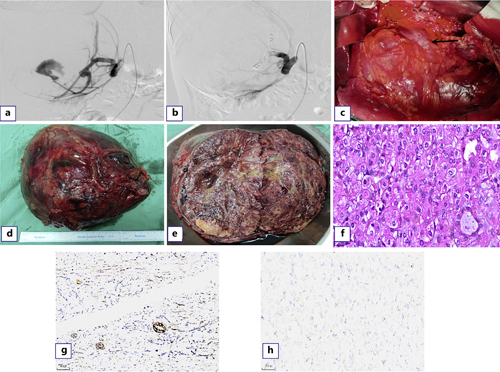
For this patient, open radical resection of the right kidney was initially considered, and selective and super-selective embolization of the main and partial branches of the right renal artery was performed before surgery (Fig. 2a, b). During the operation, the tumor seriously adhered to the inferior vena cava (Fig. 2c), which was clearly demarcated from other tissues and organs. The liver was significantly moved up due to the tumor pressure, but no metastasis was observed. Para-aortic lymph node dissection was performed during the operation. During this period, the patient’s blood pressure remained stable. Due to preoperative interventional embolization, the patient had less surgical bleeding (about 130 mL) and a clear surgical field, which took 170 min.
The gross specimen showed that the tumor was 205 × 195 × 95 mm in size, round in shape, with a complete capsule and brown color (Fig. 2d). Inside the tumor, it was gray yellow and gray red, soft and partially translucent, and the kidney was severely squeezed to one side (Fig. 2e). HE staining of pathological sections showed that tumor cells were distributed in glandular or solid form, with eosinophilic cytoplasm, oval or polygonal karyotype, fine chromatin, low ISUP grading, and no apparent nucleoli (Fig. 2f). Immunohistochemistry indicated that SDHB antibody was positive in the remaining renal tubules (Fig. 2g). SDHB was lost in tumor cells (Fig. 2h). The patient was diagnosed as SDH-deficient RCC with no evidence of tumor metastasis (including right perirenal fat, adrenal gland, and 25 para-aortic lymph nodes) on pathologic examination, and the clinicopathological stage was pT2b with significant infarction and necrosis. The patient received routine treatment and nursing after surgery but did not receive radiotherapy, chemotherapy, or other special treatments according to the pathological stage. The patient was discharged 8 days after surgery in good condition, and no evidence of metastasis or recurrence was found after 10 months of follow-up.
Discussion
SDH is a critical enzyme in the tricarboxylic acid cycle that converts succinic acid to fumaric acid and plays a role in the mitochondrial electron transport chain. It consists of four subunits: SDHA, SDHB, SDHC, and SDHD. Immunophenotypically, there are molecular changes secondary to SDH holoenzyme subunits. SDH-deficient RCC is a new subtype of RCC included in 2016 WHO classification of renal tumors. It was first recognized as RCC in 2004. The absence of SDHB immunohistochemical staining is a marker of SDH-deficient RCC. SDH-deficient RCC is a very rare aggressive subtype of RCC, accounting for approximately 0.05–0.2% of all RCCs [1‒3]. The mean age of SDHB-RCC is 47 years, with a median age of 48 years, ranging from 40 to 53 years, with a slight male predominance. Because it is often asymptomatic in humans, it is often found by accident. SDH-deficient RCC has no noticeable imaging features and is difficult to distinguish from other renal tumors. Moreover, most cases occur in young and middle-aged people. Even without a family history, immunohistochemical staining of SDHB should be performed in suspected cases, especially in patients under 45 years old. Surgical intervention is recommended as soon as the tumor is detected, rather than active surveillance alone. Nephron-sparing surgery is usually an option when the tumor is present and at an early stage. Radical nephrectomy is recommended when the tumor is in the middle stage. For advanced disease, FDG-PET is recommended. Although SDHB mutations are aggressive, unless there is evidence of sarcomatoid lesions, most patients have a good prognosis after surgical treatment [4‒6]. With longer follow-up, bilateral renal tumors were found in 26% of patients and metastatic disease in 15% [7, 8]. This disease is rare, and no recommended radiotherapy or chemotherapy strategy is currently available. SDH-deficient tumors tend to be resistant to cytotoxic chemotherapy, radiation therapy, and many targeted therapies, as demonstrated in a clinical trial of guadecitabine in SDH-deficient tumors [9]. However, it has been suggested that pathological variant morphology of cancer cells, coagulative necrosis or sarcomatoid changes, and high ISUP-grade nucleolar features are associated with more aggressive behavior. The patient reported in this study had pathologically low ISUP-grade nucleolar features (Fig. 2f) with infarction and necrosis, but this may have been related to the arterial embolization that was performed before the procedure. Therefore, how to determine the need for postoperative radiotherapy or chemotherapy and what specific drugs to use based on pathological evidence is an urgent problem to be solved.
In order to reduce intraoperative bleeding and improve surgical safety, selective or super-selective embolization of the main and branch of the right renal artery was used before surgery because preoperative imaging examination suggested large tumor volume, abundant blood supply, and existing hemorrhage. There has been no report on SDH-defective RCC with good therapeutic effect achieved by preoperative renal artery embolization (RAE). When selective embolization was performed, RAE had minimal effect on renal function [10]. In order to avoid excessive embolization resulting in difficulty in distinguishing the anatomical position of vessels during the operation, we only embolized the arteries shown in the figure while reserving the blood supply of other renal artery branches (Fig. 2a, b). Due to equipment problems in our institution, we had to perform RAE 18 h before surgery, resulting in severe pain after embolization, continuous analgesia with opioid drugs, and uncontrolled elevated blood pressure. Previous studies have suggested that intervention 3–4 h before surgery is appropriate, but using RAE as an adjunct to cancer surgery remains controversial [10, 11]. Many urology societies have not included embolization as a routine treatment for RCC due to the lack of randomized, large, prospective trials of embolization and non-embolization outcomes. In recent years, along with the advance in RAE technology and improved embolic material, a growing number of studies have shown that preoperative RAE in the surgical treatment of locally advanced large renal tumors has significant advantages, it can largely reduce intraoperative blood loss and blood transfusion, and improve the safety of surgery; we share the cases that also embody the advantages of RAE. Thus, prophylactic embolization may make some unresectable renal masses resectable and provide urologists with a reliable option for treating giant RCC [12, 13].
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines. And written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by Grant No. 202105AF150063 from the Yunnan Provincial Science and Technology Department Zhangqun Ye Expert’s Workstation.
Author Contributions
Xia Sun and Ziye Huang: drafting of the manuscript; Guang Wang: conception and design; Pei Li, Bowei Yang, and Tianyun Wang: acquisition of data; Jiongming Li: review.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.