Abstract
Introduction: There are various doses, durations, and strains of bacillus Calmette-Guérin (BCG) intravesical instillation therapy, but optimal treatment has not yet been established. We retrospectively investigated the efficacy and safety of low-dose BCG therapy for non-muscle-invasive bladder cancer (NMIBC) and carcinoma in situ (CIS) in a multicenter study. Methods: From 1991 to 2019, 323 patients who received BCG therapy to prevent recurrence of NMIBC were analyzed as group A. Similarly, 147 patients who received BCG therapy for the treatment of CIS were analyzed as group B. Patients received low- or full-dose Tokyo-172 strain or full-dose Connaught strain, and the three strains were compared. Survival curves were estimated by the Kaplan-Meier method, and independent risk factors for intravesical recurrence were examined by multivariate logistic regression. Results: Recurrence-free survival (RFS) in group A was significantly better for the Connaught strain than the low-dose Tokyo-172 strain (p = 0.026), but not between the low- and full-dose Tokyo-172 strains (p = 0.443). RFS of group B, cancer-specific survival, and progression-free survival in both groups did not show statistically significant differences. Logistic analysis of group A showed that for intravesical recurrence, only pT1 was a significant risk factor, and there were no differences between the BCG strain and dose and no significant factors in group B. There were also no differences in the completion rate in both groups, but adverse events such as urinary frequency and feeling of residual urine were significantly lower with the low-dose Tokyo-172 strain. Conclusion: There was no difference in efficacy between the low- and full-dose Tokyo-172 strains, but to minimize adverse events, the low-dose Tokyo-172 strain may be worth considering.
Introduction
Worldwide, bladder cancer (BC) is the tenth most commonly diagnosed cancer. The worldwide age-standardized incidence rate (per 100,000 person/years) is 9.5 in men and 2.4 in women [1]. Approximately 75% of patients initially diagnosed with BC present with disease confined to the mucosa (Ta, carcinoma in situ [CIS]) or submucosa (T1) and are primarily treated with transurethral resection of bladder tumor (TURBT) [2]. Although TURBT itself can completely eradicate a Ta/T1 tumor, these tumors commonly recur and can progress to muscle-invasive bladder cancer (MIBC). If it would progress to MIBC, radical cystectomy (RC) would be recommended as the standard treatment option. RC with urinary diversion may show one of the disadvantages of severely deteriorating quality of life. Furthermore, metastatic BC has a poor prognosis, with a 5-year overall survival rate of 13–15% after systemic chemotherapy [3]. Stratifying patients into low-, intermediate-, and high-risk groups using the European Organization of Research and Treatment of Cancer (EORTC) scoring system [4], which can estimate the risk of recurrence and progression after TURBT, is pivotal to the recommendation of adjuvant treatment. Intermediate- and high-risk non-MIBC (NMIBC) have high recurrence rates ranging from 24 to 78% and a high potential risk of 1–45% for progressing to MIBC [5].
Intravesical instillation of bacillus Calmette-Guérin (BCG) is currently the most effective adjuvant therapy for preventing intravesical recurrence and progression after TURBT of patients with intermediate- to high-risk NMIBC and CIS [2, 6]. Reports on intravesical instillation of BCG have shown differences in dose, strain, schedule, and duration, but optimal treatment has not yet been established. Irie et al. [7] showed the sufficient prophylactic efficacy and safety of low-dose BCG for NMIBC using the Tokyo-172 strain (Japan BCG Laboratory, Tokyo, Japan). BCG intravesical instillation therapy has two purposes: prevention of bladder recurrence for NMIBC (Ta/T1) and treatment of CIS, and it is important to distinguish between them. We retrospectively investigated the treatment efficacy and safety of low-dose BCG intravesical instillation therapy for NMIBC (Ta/T1) and CIS in a multicenter study.
Patients and Methods
Patient Selection
This study was a medical record-based retrospective study. From April 1991 to September 2019, 710 patients with primary or recurrent BC received intravesical instillation of BCG at eight hospitals affiliated with Kitasato University (Tokyo, Japan). Among them, 5 cases of low risk, 5 cases of pT2, and 67 cases with a previous history of upper tract urothelial carcinoma were excluded. Of the 436 patients who received BCG intravesical instillation therapy to prevent recurrence of NMIBC (pTa/pT1), 86 patients who received maintenance therapy and 27 patients who received second BCG intravesical instillation therapy were excluded, and the remaining 323 cases were analyzed as group A. Similarly, of the 197 patients who received BCG intravesical instillation therapy for the treatment of CIS, 23 patients who received maintenance therapy and 27 patients who received second BCG intravesical instillation therapy were excluded, and the remaining 147 cases were analyzed as group B.
Treatment Schedule and Follow-Up
All visible tumors were completely resected by TURBT and pathologically identified as NMIBC and were categorized as either intermediate or high risk according to the EORTC risk table. All primary or recurrent biopsy-proven CIS were analyzed as group B, irrespective of concomitant Ta or T1 tumors, which were completely resected. We used two types of BCG strains, Tokyo-172 and/or Connaught (Sanofi, Paris, France), but the Connaught strain stopped being supplied as of September 2017. The Tokyo-172 strain was used at either full dose (80 mg) or low dose (40 mg), the Connaught strain was used at full dose (81 mg), and the BCG strains or dose were selected by the urologist in charge. First, intravesical instillation of BCG was initiated within 3 months after TURBT. A dose of 40 or 80 mg Tokyo-172 strain or 81 mg Connaught strain suspended in 40 mL normal saline was administered intravesically, retained for 2 h, and then voided. Intravesical instillation of BCG was administered once weekly for 6–8 weeks as induction therapy, and no subsequent instillation of BCG or chemotherapeutic agents was administered to any patient in this study. Intravesical recurrence was examined by urinary cytology and cystoscopy every 3 months for the first 2 years and every 6 months thereafter, and biopsy or TURBT was performed when needed. Adverse events (AEs) during intravesical instillation of BCG were analyzed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (ver. 4.0).
Statistical Analyses
Statistical analyses of the differences related to categorical outcomes were determined by the χ2 test, and continuous variable outcomes were compared with the Kruskal-Wallis test. Recurrence-free survival (RFS), progression-free survival (PFS), and cancer-specific survival from the first intravesical instillation of BCG were calculated by medical records, and survival curves were estimated by the Kaplan-Meier method and compared with the log-rank test. For intravesical recurrence, the variables were age, sex, previous NMIBC history, intravesical chemotherapy, tumor grade, pT stage, European Association of Urology classification, and BCG strain/dose. Independent risk factors for intravesical recurrence were examined by univariate and multivariate analyses using logistic regression. All statistical analyses were performed using Stata for Windows (version 13; StataCorp, Chicago, IL, USA). p < 0.05 was considered statistically significant.
Results
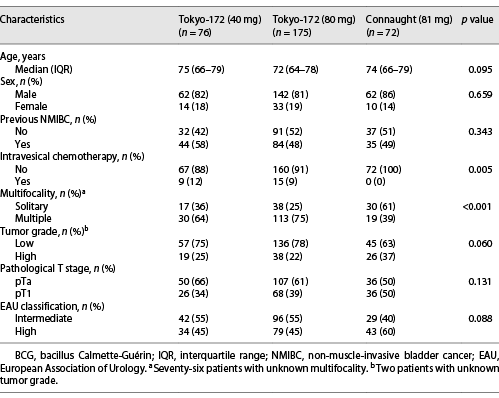
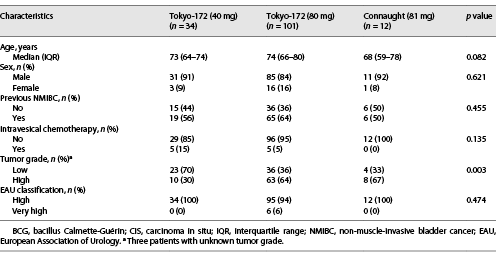
Patients’ characteristics in the NMIBC (group A) and CIS (group B) groups are shown in Tables 1, 2. The median age of group A and group B was 73 years (interquartile range [IQR] 65–79 and 64–79 years, respectively), and the median follow-up periods were 30.1 months (IQR 13.5–57.4 months) and 24.9 months (IQR 10.8–40.5 months), respectively. The median number of BCG therapies in group A was 6.4 instillations (range 1–8) for the low-dose Tokyo-172 strain, 6.3 instillations (range 1–8) for the full-dose Tokyo-172 strain, and 6.4 instillations (range 1–8) for the Connaught strain. In group B, there were 6.7 instillations (range 4–8), 7.0 instillations (range 1–8), and 6.5 instillations (range 4–8), respectively.
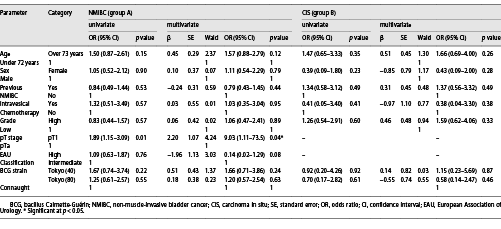
The 5-year RFS of group A was 62.0%, 71.4%, and 80.9% for the low-dose Tokyo-172, full-dose Tokyo-172, and Connaught strains, respectively (p = 0.068; Fig. 1a). There was a significant difference in bladder recurrence (p = 0.026) between the low-dose Tokyo-172 and Connaught strains, but no significant difference between the low-dose versus full-dose Tokyo-172 strains and full-dose Tokyo-172 strain versus Connaught strain (p = 0.443 and p = 0.068, respectively). The 5-year RFS of group B was 68.1%, 58.7%, and 68.2% for the low-dose Tokyo-172 strain, full-dose Tokyo-172 strain, and Connaught strain, respectively (p = 0.942; Fig. 1b). Multivariate logistic regression analyses showed that only pT1 (odds ratio [OR] 9.03, p = 0.040) was an independent predictive factor for intravesical recurrence in group A, and there were no differences between the BCG strain and dose and no significant factors in group B (Table 3).
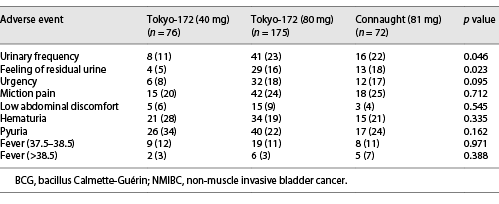
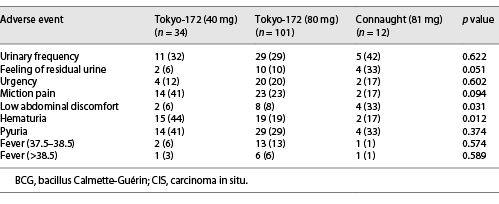
The AEs in groups A and B are shown in Tables 4, 5, respectively. In group A, urinary frequency (11%, p = 0.046) and feeling of residual urine (5%, p = 0.023) were significantly lower with the low-dose Tokyo-172 strain. In group B, low abdominal discomfort (6%, p = 0.031) was significantly lower with the low-dose Tokyo-172 strain, but hematuria (17%, p = 0.012) was significantly lower with the Connaught strain. In group B, it was difficult to determine whether the AEs were BCG or CIS related. However, the percentage of patients who could be administered at least six doses in group A was 93.4% for the low-dose Tokyo-172 strain, 90.8% for the full-dose Tokyo-172 strain, and 94.4% for the Connaught strain and in group B was 91.2%, 94.1%, and 91.7%, respectively. There were no differences in completion rate between groups.
Discussion
Preventing intravesical recurrence and reducing progression after TURBT are important issues in managing NMIBC. For that purpose, intravesical instillation of BCG is currently the most effective immunotherapy; however, there are various strains, instillation doses, schedules, and durations, and optimal treatment has not yet been established. In addition, patients with BCG unresponsive disease are unlikely to respond to further BCG therapy, and RC is the standard recommended option. However, RC involves urinary diversion and has its disadvantages. Some patients refuse RC or want repeat BCG therapy. Recently, systemic immune checkpoint inhibitor (ICI) therapy has been studied as second-line treatment after BCG failure, but it has not yet been established.
For the dose and duration of BCG intravesical instillation therapy, the Club Urológico Español de Tratamiento Oncológico study enrolled 500 NMIBC patients including 39 CIS patients (7.8%); patients were randomly assigned to receive BCG intravesical instillation therapy (Connaught strain) at a standard dose (81 mg) or one-third low dose (27 mg) once weekly for 6 weeks and thereafter once every 2 weeks for a total of six times [8, 9]. Similar results were observed with low-dose BCG intravesical instillation therapy regarding recurrence and progression and with significantly less toxicity than the standard dose. However, in patients presenting with multifocal tumors, BCG intravesical instillation therapy at a standard dose was more effective against recurrence than low-dose BCG, and there was a better trend in patients with high-risk NMIBC. In the EORTC30962 trial, intermediate- and high-risk NMIBC patients were randomized to one of four BCG therapy (TICE strain) schedule groups: one-third dose for 1 year, one-third dose for 3 years, full dose for 1 year, and full dose for 3 years [10]. The EORTC30962 trial did not find any differences in toxicity between one-third and full-dose BCG intravesical instillation therapy. However, one-third dose of BCG intravesical instillation therapy was associated with a higher recurrence rate, especially when given for only 1 year. Agrawal et al. [11] conducted a randomized controlled trial (RCT) comparing three different BCG doses (Danish 1331; 40, 80, 120 mg) in NMIBC without CIS, similar to group A in our study. All patients received a maintenance dose once monthly for 1 year. There was no significant difference in recurrence rate (20%, 25%, and 20%, respectively; p > 0.05) and no disease progression was observed, but there was a significant difference (30%, 41.7%, and 70% respectively; p < 0.01) in local toxicity. Even with the Tokyo-172 strain, a comparison of low-dose (40 mg) and full-dose (80 mg) induction therapy showed that low-dose BCG therapy was not inferior in preventing recurrence, and the incidence of side effects was reduced [7, 12]. In a meta-analysis, low-dose BCG intravesical instillation therapy had the same effect as full-dose BCG intravesical instillation therapy in preventing progression, and a decreased incidence of AEs was observed, but there were varying results regarding the effect of low-dose BCG on preventing recurrence [13‒15]. In our study, the low- and full-dose BCG Tokyo-172 strains were comparable for RFS, PFS, and cancer-specific survival for both NMIBC (group A) and CIS (group B). In addition, the low frequency of AEs may be more useful for cases where it is desirable for AEs to be suppressed.
Next, we assessed different BCG strains. Two prospective studies compared the differences between BCG strains [16, 17]. One RCT compared the Tokyo-172 strain with the Connaught strain and the other RCT compared the Connaught strain with the TICE strain. There were no significant differences in RFS (p = 0.748) and AEs between the Tokyo-172 strain and Connaught strain. However, in the RCT comparing the Connaught strain and TICE strain, the 5-year RFS was significantly better for the Connaught strain (74% and 48%, respectively; p = 0.0108). Boehm et al. [18] conducted a systematic review and network meta-analysis of trials evaluating the outcome, including 10 different BCG strains, with intravesical chemotherapy as the common comparison. Among them, the Tokyo-172 strain (OR = 0.39, 95% confidence interval [CI]: 0.16–0.93), Pasteur strain (OR = 0.49, 95% CI: 0.28–0.86), and TICE strain (OR = 0.61, 95% CI: 0.40–0.93) were significantly better than intravesical chemotherapy in preventing recurrence. However, no BCG strain demonstrated significant superiority compared to any other strain at preventing recurrence in this network meta-analysis. Inamoto et al. [19] conducted a prospective study comparing the low-dose Tokyo-172 strain (40 mg) with the standard-dose Connaught strain (81 mg) and reported that neither RFS nor AEs were significantly different between treatment groups. However, in our retrospective study, the low-dose Tokyo-172 strain led to a worse RFS than the Connaught strain due to various biases. However, the completion rate of the Connaught strain was very different from the 70% (Inamoto et al.) versus 94% (our study), which may have affected the difference in RFS.
Regarding the administration schedule and optimal length of BCG intravesical instillation therapy, European Association of Urology guidelines reported that for optimal efficacy, BCG must be given in a maintenance schedule [2]. Lamm et al. [20] reported, in the Southwest Oncology Group (SWOG) 8507 study the efficacy of 3-year BCG maintenance therapy for recurrence of Ta/T1 and CIS. The maintenance therapy schedule was intravesical instillation once a week for 3 weeks at months 3, 6, 12, 18, 24, 30, and 36 for a total of 27 instillations after induction therapy. The median RFS was twice as long for maintenance therapy than for only induction therapy (p < 0.0001), and patients had a significantly longer PFS (p = 0.04). However, due to AEs, only 16% of patients were able to complete the 36-month treatment schedule. Many studies have subsequently reported on the optimal length of maintenance therapy. Hinotsu et al. [21] reported the efficacy of 18-month BCG maintenance therapy for Ta or T1 cases excluding CIS in a randomized study. Their maintenance therapy schedule consisted of three consecutive intravesical instillations once a week, for a total of 18 instillations at 3, 6, 12, and 18 months after induction therapy. The maintenance therapy significantly prolonged RFS, and 41.7% of patients were able to complete that schedule. The KURG study [22] was BCG maintenance therapy for recurrent or multiple Ta/T1 or CIS patients with the Connaught strain. The schedule was 6 weeks of induction therapy followed by once a week for 2 weeks at months at 3, 6, 12, 18, 24, 30, and 36, for a total of 20 instillations in 3 years. Because in the KURG study, the number of maintenance instillations was reduced from three to two, patients tolerated BCG treatment well, and the completion rates were high during the 3 years of treatment, with cancer control rates comparable to other reports [20, 23]. Koguchi et al. [24] reported that maintenance therapy with the low-dose BCG Tokyo-172 strain (40 mg) showed acceptable efficacy and safety on the same schedule as the KURG study. In our study, we excluded patients who received BCG maintenance therapy and examined only those who received BCG induction therapy because the BCG maintenance therapy schedule was different for each institution. However, the effectiveness of BCG maintenance therapy has basically been shown, and it is important to consider further appropriate schedules and doses in the future.
Finally, we evaluated the treatment of BCG failure. If CIS remains after the first induction of BCG therapy, complete response (CR) can be expected in 40–60% of patients and repeat BCG therapy may be a treatment option [25]. The Tokyo-172 strain is the only BCG strain that can be used in Japan, and there was no difference in efficacy between the low- and full-dose Tokyo-172 strain in our study. Repeat BCG therapy is a necessary treatment option, and low-dose BCG therapy may be preferred to reduce AEs and increase completion rates. However, the majority of BCG failures are BCG unresponsive. BCG-unresponsive disease, which includes BCG-refractory and BCG-relapsing categories (within 6 months of last BCG exposure), is a subgroup of patients with high risk of recurrence and progression in whom repeat BCG therapy is not a feasible option [26]. Nevertheless, repeat BCG therapy or novel treatment options are needed for patients who refuse RC or who have a strong desire for bladder preservation. Currently, a number of studies on systemic ICI therapy for BCG-unresponsive disease are active. The KEYNOTE-057 study was a phase 2 study evaluating the efficacy and safety of pembrolizumab for BCG-unresponsive disease [27]. Pembrolizumab monotherapy was tolerable and showed promising antitumor activity in patients with BCG-unresponsive disease (CR was 41% at 3 months). Similarly, the phase II SWOG S1605 study with atezolizumab in patients with BCG-unresponsive high-risk NMIBC showed a CR of 41.1% in 3 months. In addition, ICIs such as avelumab, durvalumab, and nivolumab are currently widely used for NMIBC, especially BCG-unresponsive disease [28].
This study had some limitations. First, some AEs may not have been identified because this was a retrospective, multicenter study. Second, there was a treatment selection bias because the choice of BCG strain and dose were selected by the urologist in charge rather than being a double-blind study. Finally, the number of therapeutic BCG groups for CIS was small; thus, extensive research is needed in the future.
Conclusion
The Connaught strain may be superior to the others on RFS. But currently, since the Connaught strain stopped being supplied, the Tokyo-172 strain is the only BCG strain that can be used in Japan. There was no difference in efficacy between the low- and full-dose Tokyo-172 strains, but to minimize AEs, the low-dose Tokyo-172 strain may be worth considering. Further studies are needed for more appropriate case selection.
Acknowledgments
We would like to thank Drs. Yoshinori Taoka, Takuji Utsunomiya, Hideshige Koh, Daisuke Matsuda, Norihiko Okuno, Junichiro Ishii, and Takahiro Hirayama for their invaluable help with the data collection.
Statement of Ethics
This study was conducted in accordance with the Declaration of Helsinki and its amendments and with approval of the Ethics Committee of Kitasato University School of Medicine and Hospital (Approval No. B18-264). Due to the retrospective design of the study, the Ethics Committee waived the requirement for written informed consent. The patients had the option of opting out of the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There are no funding sources to declare.
Author Contributions
Takashi Tachibana: project development, manuscript writing, and submission. Masaomi Ikeda: data analyses and manuscript writing and editing. Soichiro Shimura, Noriyuki Amano, Yasukiyo Murakami, Yasufumi Yamada, Dai Koguchi, Ryota Maeyama, Mizuho Kawamura, Yusuke Sakata, and Masahiro Hagiwara: data collection. Kazumasa Matsumoto: project development and manuscript editing. Masatsugu Iwamura: project development.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.