Abstract
Introduction: The aim of the study was to report our initial experience of robot-assisted kidney transplantation (RAKT) with a modified hypothermia technique. Methods: Between March 2018 and May 2020, 12 patients with end-stage renal disease underwent RAKT at the Chinese PLA General Hospital, and a modified regional hypothermia was implemented by wrapping the kidney in a sealed plastic jacket filled with ice slush. Baseline, surgical, and functional outcomes were analyzed. Results: All surgeries were successfully performed. The mean operative time was 180.5 min, with a mean console time of 133.3 min. Mean warm ischemia, cold ischemia, and rewarming times were 1.5, 135.1, and 48.4 min, respectively. The median blood loss was 50 mL, and the median hospital stay was 9.5 days. No complications were observed. The mean serum creatinine levels were 119.4, 100.5, 108, and 108.5 μmol/L 7 days, 1 month, 6 months, and 1 year postoperatively, respectively. All patients and grafts survived at a median follow-up of 32.2 months. Conclusion: RAKT is a safe and feasible procedure for surgical teams with expertise in open kidney transplantation and robotic surgery. Our modification of the hypothermia technique can maintain the kidney at a constant low temperature without repeatedly adding ice and prevent the complication of paralytic ileus.
Introduction
Due to the greater survival rate and improved quality of life compared with those of hemodialysis, kidney transplantation (KT) is considered the preferred treatment for end-stage renal disease (ESRD) [1]. KT recipients are fragile and immunocompromised after surgery, so minimally invasive surgery can undoubtedly reduce the risk of complications such as wound infection and delayed healing in these patients [2]. However, due to the difficulties in laparoscopic vascular anastomosis and intracorporeal graft hypothermia, the open technique has remained the gold standard in KT for the past 60 years.
With the development of the da Vinci surgical system, the learning curve for laparoscopic anastomosis has shortened with robotic assistance [3]. Hoznek et al. [4] reported the first robotic approach in KT in 2002 [4], followed by several case reports of robot-assisted KT (RAKT) in 2010 and 2011 [5, 6]. However, all these procedures were performed without intraoperative renal cooling. In 2014, Menon et al. [7] reported the use of pure RAKT with regional hypothermia and standardized the procedure [7‒9]. Since then, an increasing number of clinical centers around the world have begun to adopt RAKT and obtained satisfactory results [10‒20].
On this basis, our center performed the first RAKT in China on March 2018 and made some modifications in terms of graft hypothermia. In the present study, we aimed to report our initial experience in China with RAKT from living donors in 12 patients. Surgical and functional outcomes at a minimum 1-year follow-up were also determined.
Methods
Patients
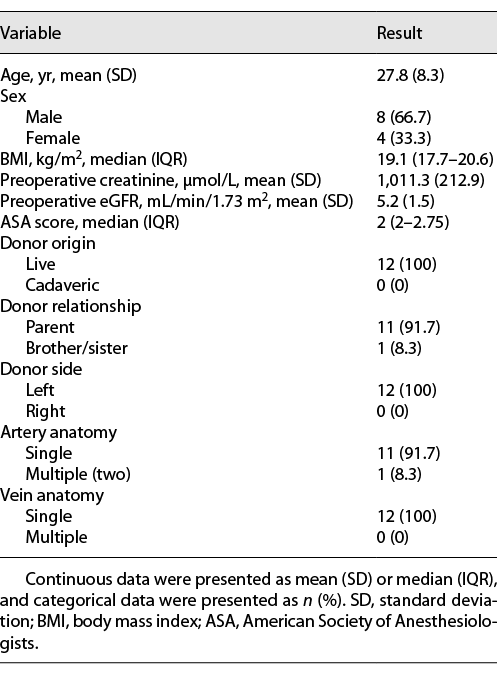
The records of 12 patients with ESRD who underwent RAKT at the Chinese PLA General Hospital between March 2018 and May 2020 were prospectively collected. The procedures were performed by the same robotic and transplant team. All graft kidneys were from the left donor side and harvested from laparoscopic living donor nephrectomies. CT and color Doppler ultrasonography were used to determine the vessel conditions of both the recipients and donors. Table 1 lists the baseline characteristics of the patients. The exclusion criteria were malignancies, positive virology, severe comorbidities, previous major abdominal surgeries, and vessel conditions not suitable for KT [12]. This study was approved by the Ethics Committee of the Chinese PLA General Hospital, and written informed consent was obtained from all patients.
Surgical Technique
Modification of Regional Hypothermia Technique
After laparoscopic nephrectomy, the harvested kidney was perfused with 4°C hypertonic purine citrate solution, and the renal vessels and ureter were trimmed for later anastomosis. Instead of gauze, the graft kidney was wrapped in a plastic jacket filled with ice slush with an opening to facilitate the exposure of the renal vessels and vascular anastomosis (Fig. 1). In this way, the isolation effect of the plastic material could reduce heat exchange between the kidney and the environment, and the better sealing of the plastic jacket could prevent some of the melting ice from spreading into the abdominal cavity.
Robotic Procedure
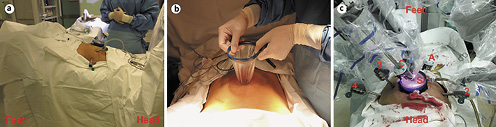
The procedure was based on the Vattikuti Urology Institute–Medanta technique [8]. The patient was placed in a lithotomy position with a 20° Trendelenburg tilt (Fig. 2a). A 5–6 cm periumbilical vertical incision was made, and a single-port platform (Ningbo Senscure Biotechnology Co., Ningbo, China) was inserted (Fig. 2b). A 12-mm port connected to the single-port platform was used as the camera port. Two 8-mm robot arm ports were placed at the right and left paramedian lines just below the umbilicus level, and the fourth 8-mm robot arm port was placed 8 cm lateral to the left robot arm port. A 12-mm assistant port was placed 8 cm below the right robot arm port along the right midclavicular line. The robot (da Vinci® SiTM, USA) was docked between the legs (Fig. 2c).
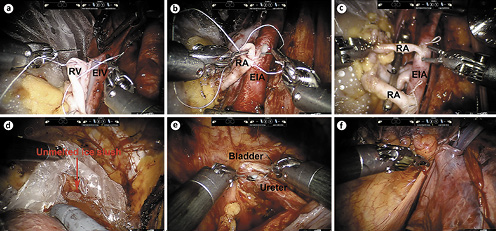
A transverse peritoneal incision was made, and the Retzius space was entered. The bladder was dropped down, and the right external iliac vessels were skeletonized for vascular anastomosis. After the graft kidney was fully prepared, the robotic camera arm and single-port cap were removed, and the packed kidney was introduced through the periumbilical vertical incision with the lower pole of the kidney facing down and the renal hilum facing the right external iliac vessels. Then, the robot was redocked, and the fourth robotic arm was used to hold the kidney in place. As previously described [8], end-to-side continuous anastomoses of the renal vein with the external iliac vein and of the renal artery with the external iliac artery were performed (Fig. 3a, b). For the sole graft with double renal arteries, both renal arteries were anastomosed with the external iliac artery (Fig. 3c). Then, the blood supply to the kidney was restored. Of note, the ice slush in the plastic jacket was not fully melted during the rewarming time (RT) (Fig. 3d). The ureteroneocystostomy was performed with the modified Lich-Gregoir technique (Fig. 3e), and a double-J stent was inserted into the ureter. For extraperitonealization of the kidney, the graft kidney was moved to the right iliac fossa prior to ureteroneocystostomy to check for any potential kinking or overtension of the vessels. If there was no kinking or overtension, after the ureteroneocystostomy, the kidney was extraperitonealized in the right iliac fossa by closing the incised peritoneum (Fig. 3f).
Postoperative Care and Data Collection
Standard postoperative care, including antibiotics, nutrition, pain control, and early ambulation, was provided to all patients. The immunosuppression regimen was administered as previously described [20]. Vascularization of the graft kidney was examined by color Doppler ultrasound intraoperatively and on postoperative day 1. The double-J stent was removed cystoscopically on postoperative day 30. The data for surgical, functional, and survival outcomes were prospectively collected, and serum creatinine was obtained 7 days, 1 month, 6 months, and 1 year postoperatively. Warm ischemia time was defined as the time between renal artery clamping in the donor and cold perfusion of the graft; cold ischemia time was defined as the duration of cold storage of the graft before introduction into the recipient’s abdominal cavity; and RT was defined as the time between graft insertion into the recipient and graft revascularization. Estimated glomerular filtration rate (eGFR) was calculated using the modified diet in renal disease equation [21], and complications were graded according to the Clavien-Dindo system [22].
Results
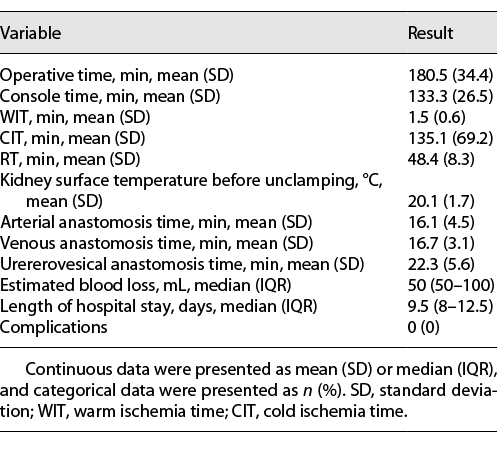
All procedures were successfully completed without open conversion. Surgical data are reported in Table 2. The mean operative time was 180.5 ± 34.4 min, and the mean console time was 133.3 ± 26.5 min. The mean warm ischemia time was 1.5 ± 0.6 min, the mean cold ischemia time was 135.1 ± 69.2 min, and the mean RT was 48.4 ± 8.3 min. The mean anastomosis times for the arteries, veins, and ureteroneocystostomy were 16.1 ± 4.5, 16.7 ± 3.1, and 22.3 ± 5.6 min, respectively. During the rewarming process, the ice slush on the surface of the kidney did not melt completely, and the mean surface temperature of the kidney was 20.1 ± 1.7°C. The median estimated blood loss was 50 mL (interquartile range [IQR] 50–100), and no patients needed transfusion. The median hospital stay was 9.5 days (IQR 8–12.5). No perioperative or late complications occurred.
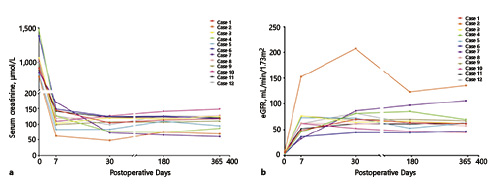
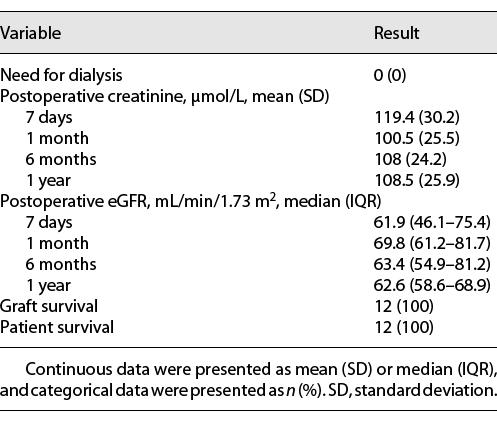
Color Doppler ultrasound showed good vascularization of the graft kidneys in all patients intraoperatively and on postoperative day 1. Postoperative functional and survival data are listed in Table 3. A significant improvement in renal function was observed as time went on, and no patients needed dialysis. Specifically, the mean serum creatinine was 108.5 ± 25.9 μmol/L 1 year postoperatively, and the median eGFR at 1 year postoperatively was 62.6 mL/min/1.73 m2 (IQR 58.6–68.9). The serum creatinine and eGFR changes for each individual patient are shown in Figure 4, and the cosmetic result was satisfactory. All patients and grafts survived with a median follow-up time of 32.2 months (range 16.1–42.7).
Discussion
Open kidney transplantation (OKT) has long been considered the gold standard treatment for patients with ESRD [1]. Until recently, with the development of minimally invasive technology, especially robotic approaches, OKT has been challenged by the alternative procedure of RAKT [4‒9, 12, 13, 16‒20]. RAKT was first described by Hoznek et al. [4] in 2002, and several explorations in this field followed thereafter [5, 6]. In 2014, Menon et al. [7] reported RAKT with regional hypothermia and made the procedure standardized and systematic through a series of clinical studies [7‒9], which marked a milestone in minimally invasive KT. Since then, various RAKT series have been published with satisfactory results [10‒20], and our study represents the first experience with RAKT in China.
Compared with those of OKT, the advantages of RAKT are obvious: fewer surgical site infections, minimal postoperative pain, shorter convalescence periods, and better cosmetic results [8, 12]. These benefits were better achieved in obese KT recipients, some of whom were even excluded from traditional OKT [11, 19, 20]. However, the primary concern in KT is always graft and patient survival. Several high-volume centers have reported satisfactory functional outcomes for RAKT patients. Breda et al. [12] reported a multicenter RAKT series of 120 patients, and the mean serum creatinine level at 1 month was 130 μmol/L. For Menon’s IDEAL PHASE 2a study of 25 patients with a 6-month follow-up, the mean serum creatinine level was 1.1 mg/dL (97.2 μmol/L) [8]. In our present study of 12 patients, the mean serum creatinine levels at 6 months and 1 year were 108 and 108.5 μmol/L, respectively, which were comparable or even better than those in published RAKT series [8, 10, 12‒14, 16‒20]. When compared with those of the open approach, the functional outcomes of RAKT are similar, although the operative time and RT were somewhat longer [13, 18, 23]. Even so, the largest European multicenter study from the European Robotic Urology Section – RAKT group revealed that neither surgical time nor RT were correlated with postoperative serum creatinine levels [17]. In terms of complications, Tugcu et al. [13] reported a lower complication rate for RAKT than for OKT, especially for wound infections and lymphoceles. For arterial graft thrombosis and delayed graft function, the complication rates were comparable between different groups undergoing RAKT and standard open surgery in previous publications [12, 24, 25]. In our study, no obvious complications were observed.
As the RT of RAKT was relatively longer than that of the open technique, the maintenance of intracorporeal graft hypothermia is a key factor in protecting renal function. Menon et al. [8] employed the method of wrapping the graft in a gauze jacket filled with an ice slush. The disadvantage of this method is that ice slush wrapped in gauze can easily melt and thus needs to be constantly added around the kidney in the rewarming process, which affects the continuity of the operation. Meanwhile, the melted ice water can flow into the abdominal cavity through the gauze jacket and cause the patients to suffer from ileus. Tugcu et al. [13] reported that 2 out of 15 RAKT patients developed paralytic ileus as a complication of adding the ice slush. We modified this technique and chose a plastic bag with better sealing to wrap the graft kidney and fill it with ice slush. First, the isolation effect of the plastic bag can reduce heat exchange between the kidney and the environment; second, even if the ice slush partially melts, it will form an ice-water mixture that will continue to surround the kidney without spreading into the abdominal cavity, maintaining the kidney at a constant low temperature and preventing paralytic ileus to a great extent. To reduce the risk of freezer burn to the kidney, we used a two-layer plastic bag and the ice slush was filled between the layers not contacting the kidney directly. We found that the ice slush around the graft kidney did not completely melt before vascularization was performed, resulting in a mean kidney surface temperature of 20.1°C during the rewarming process, so no further ice was added during our procedure, and the postoperative renal functions were also satisfactory. Moreover, no complications of paralytic ileus were reported in our cohort. Undoubtedly, the decision to add ice is also related to the surgeon’s robotic skill in performing vascular anastomosis, so for cases where rewarming is expected to take a long time, we still recommend adding ice to maintain regional hypothermia. Regarding other hypothermia techniques, Meier et al. [26] introduced an intra-abdominal cooling system to continuously cool the kidney, but his technique was still in the animal experiment phase and cannot be easily accomplished in the clinic. In contrast, our technique is easy to follow and cost effective.
Although standardized in one way or another, RAKT is still a challenging procedure for beginners. To guarantee the safety of the procedure and graft survival in the initial learning curve, several aspects should be noted. First, a surgical team with expertise in both OKT and robotic surgery is required. Prior to the first RAKT procedure in our series, over 2500 OKT operations and over 4,000 robotic surgeries were performed by our team. Moreover, the robotic surgeon in our team had performed many procedures of robotic inferior vena cava thrombectomy and gained rich experience with robotic vascular reconstruction [27, 28], which undoubtedly facilitated the arterial and venous anastomoses in RAKT. The European Robotic Urological Society RAKT group found that a minimum of 35 cases were necessary to reach reproducibility in terms of RWT, complications, and functional results [16]. Second, the recipients and donors of RAKT should be strictly selected. Due to the lack of tactile feedback in the robotic surgery system, it is difficult to assess the degree of arteriosclerosis in the recipients, which could compromise the quality of vascular anastomosis [3]. Donors with highly complex anatomies of the renal vessels are not recommended for RAKT [12]. To date, most RAKT series, including ours, have used living donor kidneys [8, 10, 14, 18]. This provides surgeons with sufficient time to make preoperative preparations to ensure the success of the procedure, and the high quality of the donor kidney can exclude nonsurgical factors from affecting the prognosis of the patients. With accumulation of experience, RAKT can also be performed with deceased donors with favorable outcomes [15].
One of the limitations of our study is the small number of cases and the short follow-up time. Although our initial experience has yielded remarkable surgical and functional outcomes, a larger series and longer follow-up time are needed to confirm its repeatability. Another limitation is the lack of a control group for OKT. A randomized controlled trial in the future is necessary to better assess the advantage of RAKT.
Conclusion
RAKT is a safe and feasible procedure for surgical teams with expertise in OKT and robotic surgery. Our modification of the hypothermia technique can maintain the kidney at a constant low temperature without repeatedly adding ice and prevent the complication of paralytic ileus. To the best of our knowledge, we present the first experience of RAKT in China with excellent outcomes. A larger cohort and longer follow-up with an open surgery control group are needed to further assess the advantages of RAKT.
Statement of Ethics
This study was reviewed and approved by the Ethics Committee of the Chinese PLA General Hospital (No. 2018090), and written informed consent was obtained from all patients.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This manuscript did not receive any funding.
Author Contributions
Xu Zhang and Jun Dong contributed to the conception and design; Yang Fan, Jianming Zhao, and Qiang Zu contributed to the drafting of the manuscript; Yu Gao and Donglai Shen contributed to the acquisition, analysis, and interpretation of data; and Qiang Zhu, Shuang Huang, and Xin Chen contributed to the administrative technical or material support.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Additional information
Yang Fan, Jianming Zhao and Qiang Zu are co-first authors.